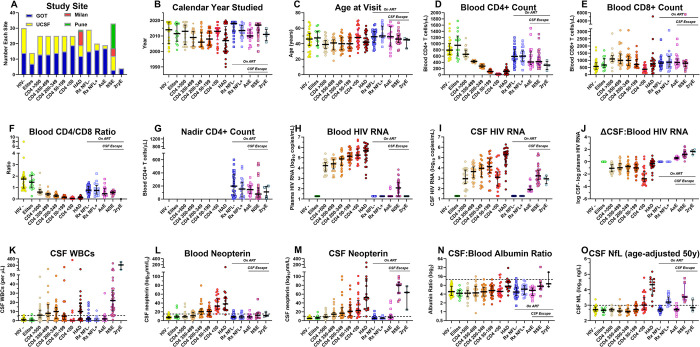
Fig 1. Study group background characteristics.
Except for the first panel (Fig 1A), the graphs (Fig 1B–1O) use the same format that is applied to subsequent figures showing the Olink-generated protein measurement profiles across the study groups. This includes the symbols and the median and IQR bars. The dashed horizontal lines in Fig 1K–1O indicate the mean +2 SD of the uninfected, seronegative (HIV-) control group in the measured variable as a rough estimate of their upper normal limit and as a visual guide to compare groups; this convention is also used in the Olink CSF protein measurement figures showing the group profiles in subsequent figures. A. Study sites. Most of the samples were obtained in the context of scheduled outpatient visits during long-term cohort studies in Gothenburg, Sweden (GOT) and San Francisco, California, USA (SF) that included lumbar punctures (LPs) as part of the study protocols (Fig 1A). This included CSF specimens from the HIV-, elite controllers (elites), five neuroasymptomatic CD4-defined groups, two ART-treated and virally suppressed groups (one with normal CSF NfL and one with elevated CSF NfL, designated as RxNFL+ and RxNFL-) and the asymptomatic escape (AsE) group. The HIV-associated dementia (HAD), neurosymptomatic escape (NSE) and secondary escape (2ryE) samples were obtained during clinical evaluations after informed consent. Additionally, the NSE and HAD groups were augmented by CSF specimens from Milan and Pune to attain a size comparable to the other main groups. When there was a larger number of CSF specimens available for a given group than required for the study, samples were chosen at random from archived collections (e.g., CD4-defined groups). An exception to this random selection was the choice of the HIV- group from GOT: all of these were from individuals taking pre-exposure prophylaxis (PrEP) because of their self-identified risk for HIV-1 infection. HIV- controls from SF were seronegative individuals from the same clinical site and demographic population as the people living with HIV (PLWH) included in the local cohort, but not on PrEP. The Rx NFL+ and the AsE groups were almost all from GOT related to the local interest in these conditions. The small number of secondary escape (2ryE) were also all from GOT. In general, the imbalances in the subject group sizes related to scarcity of available samples (e.g., elites and 2ryE, resulting in smaller sample sizes), or to inclusion of larger numbers of specimens to augment particularly important groups, including the HIV- group, related to its comparative utility, and HAD and NSE groups because of our interest in more severe HIV-1-related CNS injury. These imbalances, along with those of calendar years and subject ages (B and C below), emphasize that this exploratory study used a convenience sample rather than one that was balanced with respect to individual demographic variables. B. Calendar year. The dates of study varied among the groups. In part this reflected the history of our cohort studies in concert with the changes in acceptance and efficacy of treatment regimens over the collection period. These time variations impacted the length of untreated chronic infection, susceptibility to disease progression and presence of viral suppression among our study cohorts. For example, there were nearly 10 years separating the median years of the HAD group from both treatment-suppressed groups (RxNFL- and RxNFL+). The NSE group sampling also reflected later recognition and attention to this clinical entity. C. Age. The ages of the subjects were not specifically selected and consequently also varied among groups, with group medians ranging from 39 to 50 years with largely similar ranges. Overall, the median ages of the treated PLWHs were similar to those of the HIV-negative (HIV-) controls, while the untreated groups were generally younger. The balance of sex was not taken into account with specimen choices, so distribution also varied among the groups. D. Blood CD4+ T-lymphocytes. The median blood CD4+ T-cell count in the elites was above that of the uninfected controls and the other HIV-1-infected groups, including the two treatment suppressed groups. The blood CD4+ T-cell concentrations in the 5 groups defined by these counts show the stepwise decrease from >500 to <50 CD4+ T-cells/μL dictated by the study design. The HAD group had a median CD4+ T-cell count of 108 cells/μL that was similar to the 50–199 CD4+ group median of 106 cells/μL, in keeping with the role of advanced immunosuppression in the development of this subacute disorder and its underlying HIVE pathology. The CD4+ T-cell counts of the two treatment-suppressed groups were nearly equal, with both having blood CD4+ T-cell counts above those of the viremic CD4-defined groups except for the group with the highest blood CD4+ T-cell counts (CD4>500 group), indicating the partial CD4+ cell preservation or recovery with suppressive treatment. By contrast, the blood CD4+ T-cell counts in the three CSF escape groups were lower than the two treatment-suppressed groups, consistent with less robust recovery or ongoing loss during the escape episodes. However, these levels were not as low as in the untreated HAD group. The CD4+ blood counts of the 2ryE subjects were also low, indeed below the other two escape groups, likely contributing to their vulnerability to intercurrent infections (herpes zoster in three and HSV2 meningitis in the fourth) and factoring into their clinically-directed LPs. E. Blood CD8+ T lymphocytes. Blood CD8+ T-cell counts were on the high side of normal in the elites, rose as blood CD4+ cells decreased in the untreated CD4-defined groups except for those in the CD4 <50 group (median of 380 CD8+ T-cells per μL). They were relatively increased in the HAD group (median 750 CD8+ T-cells per μL) and in all the treated groups, including the two virally suppressed and the NSE and ASE groups (medians in 800s per μL). F. Blood CD4+/CD8+ T-cell ratio. The differences in the trajectories of the two T-cell subpopulations resulted in lowered ratios in all infected groups compared to the HIV- controls, reflecting variable reductions in CD4+ and elevations of CD8+ T-lymphocytes. Reductions were most severe in the CD4 -defined groups, most notably in those with CD4+ T-cell counts below 200 cells per μL and in the HAD group. These ratios were relatively increased in the four treated groups (0.47 to 0.77), though all remained below the level of the uninfected controls (median ratio of 1.75). Reduced ratios persisted in the two ART-suppressed groups (RxNFL- and RxNFL+) and the AsE and NSE groups. G. Nadir blood CD4+ T-lymphocytes. Nadir CD4+ T-cell counts are shown only for the ART-treated groups, since CD4+ T-cell values in the untreated groups at their study visits were generally at or near their nadirs. The treated groups showed varying degrees of presumed blood CD4+ T-cell recovery, higher in the two virally suppressed (Rx NFL- and NFL+) groups (medians of 590 and 600 CD4+ T-cells per μL) than in the CSF escape NSE and ASE groups (medians of 425 and 412 CD4+ T-cells per μL, respectively), the latter perhaps either contributing to development of CSF escape or, alternatively, reflecting an impact of escape on CD4+ T-cell dynamics. H. CSF HIV-1 RNA. Highest CSF HIV-1 RNA concentrations were present in the HAD group, while the CD4-defined groups showed the ‘inverted U’, or lymphoid, pattern of change with lower concentrations at the extremes (in the CD4+ >500 and CD4+ <50 groups) than in the middle ranges (CD4+ 350–499, 200–345 and 50–199 groups). We previously reported the association of this pattern with that of the CSF WBC counts as also noted in this study (see Fig 1K below), suggesting that these two findings were causally related [45]. In this study the correlation of CSF HIV-1 RNA to CSF WBC count across the CD4-defined groups was significant (Pearson correlation P = 0.006). This lymphoid pattern of infection and cell response likely underlies this same pattern in many of the CSF proteins included in the Olink Explore 1536 panel as shown in later figures. The CSF HIV-1 RNA concentrations of three escape groups were generally below those of the untreated groups except for the CD4 <50 group. CSF HIV-1 RNA concentrations were all below detection in the elites. I. Blood HIV-1 RNA. In the untreated individuals (not including the elites) there was a steady increase in blood HIV-1 RNA through the full range of CD4+ T-cell loss that reached its highest mean levels in the HAD group. Unlike with CSF, there was no decrement in the CD4 <50 group, showing a distinct difference between the CSF and the systemic blood viral dynamics. The presence of low-level blood HIV-1 RNA in the NSE group may have reflected systemic partial drug resistance in some or spillover of the ‘escaped’ CNS/CSF infection into the blood. J. CSF:Blood HIV-1 RNA differences. This panel shows the differences in viral loads between the two fluids (calculated as the log10 CSF HIV-1 RNA copies per mL–log10 plasma HIV-1 RNA copies per mL) and emphasizes the largely consistent relationship between CSF and blood HIV-1 RNA levels in untreated infection at blood CD4+ T-cell levels between 50 and >500 cells/μL in which the CSF concentrations were approximately 10-fold lower than those in blood, and thus maintaining a nearly 1:10 ratio of CSF to blood HIV-1 RNA. This may relate to the kinetics in the influx of infected CD4+ T-cells and of viral release into the CSF over this blood CD4+ T-cell range. This ratio was disrupted when blood CD4+ T-cells fell to <50 cells/μL and the CSF WBC count decreased to negligible levels; in this group the CSF:blood HIV-1 RNA ratio decreased nearly 10-fold to an overall ratio of <1:100. This underscores the importance of lymphocyte traffic and likely direct virus release by trafficking infected CD4+ T-cells in the determining the CSF HIV-1 levels in the neurologically asymptomatic individuals, particularly those with blood CD4+ T-cell counts above 50 cells per μL. This relationship changed markedly with the development of HAD and the underlying direct neuropathic parenchymal CNS infection, i.e., HIVE, in which the CSF HIV-1 RNA concentrations reached high levels, and the differences between it and the blood viral load decreased. Thus, in the HAD group local brain infection rather than hematogenous sources likely was responsible for the high levels of CSF HIV-1 RNA. The reversed CSF:blood HIV-1 RNA ratios in the three CSF escape groups were consonant with their definitions predicated on CSF > blood HIV-1 RNA concentrations that indicated the direct CNS sources of the measured CSF HIV-1 RNA in the face of systemic viral suppression. K. CSF WBC counts. The median CSF cell counts rose and then fell over the course of untreated infection in the CD4-defined groups. CSF WBC counts were highest in the 200–349 group and declined to lowest levels when blood CD4+ T-cells fell below 50 cells/μL, defining the lymphoid pattern. As discussed above, this likely was causally linked to the changes in CSF HIV-1 RNA in these groups. Both neurologically defined groups were associated with augmented local inflammation: CSF WBCs were elevated in HAD, and even higher in NSE (median counts of 10 and 22 cells per μL, respectively). In these two settings the CSF WBC counts likely involved a response to local CNS infection and injury. L. CSF neopterin. This pteridine is predominantly, though perhaps not exclusively, a macrophage-related activation marker [143]. CSF levels showed a steady median increase as CD4 cells declined, including the highest levels in the CD4+ <50 group among the CD4-defined sequence of groups, thus providing a prototypical example of the myeloid pattern of CSF biomarker change. CSF concentrations were even higher in the HAD group, and, notably, were highest in the NSE group, while suppressive treatment brought CSF neopterin concentrations back to near normal levels in the absence of symptomatic CSF escape. M. Blood neopterin. In blood, neopterin concentrations showed a similar, though more restricted elevation with CD4 decline but were notably increased in both the CD4 < 50 and HAD groups, presumably indicating systemic macrophage activation in these settings that either links these two sites of infection or marks parallel changes in myeloid cell populations in the CNS and systemically. In contrast to CSF, the blood neopterin was only mildly elevated in the NSE and 2ryE groups in keeping with the underlying compartmentalized CNS HIV-1 and intercurrent infections in these two groups in the face of systemic HIV-1 suppression. N. CSF: blood albumin ratio. This ratio provides an index of blood-brain barrier integrity, though with considerable individual and age-related variability [144]. In this sample set, only direct HIVE in the HAD group and, to a lesser degree, in the NSE group was associated with elevated median albumin ratios. Minor, though not significant, increases were present in the RxNFL+ group. One-way ANOVA with Tukey’s multiple comparison test found significant differences (P<0.05) only for HAD (CD4 >500: P = 0.0004; Elites: P = 0.0028; AsE: P = 0.0067; CD4 200–349: P = 0.0069; RxNFL-: P = 0.0116; CD4 50–199: P = 0.0202; CD4 350–499 P = 0.0228) and NSE (CD4 >500: P = 0.0023; Elites: P = 0.0075; AsE: P = 0.0173; CD4 200–349 P = 0.0211; HIV-1- P = 0.024; RxNFL- P = 0.0337; CD4 50–99 P = 0.0479). Other intergroup comparisons were not significant. Overall, the albumin ratio elevations were small, and likely to have had only limited, if any, impact on the CSF proteins measured in this panel. O. CSF NfL. Prior to this study, CSF NfL was measured using the UMAN ELISA method (UmanDiagnostics, Umeå, Sweden) in 209 of the 307 (71.3 percent) of the specimens. In this figure, the NfL values have been adjusted to age 50 years using the normative data and methods of Yilmaz and colleagues [68] to allow more direct subject comparisons and estimations of abnormal values. While these data were incomplete and superseded in this study by the Olink measurements performed on all samples, they guided our initial definition of the RxNFL+ group. The highest levels of NfL were in the HAD group, and there were elevated levels in a substantial proportion of the CD4 <50 group presumably related to subclinical neurological injury. Notably, the NfL elevation of the HAD group had nearly a 10-fold higher median value than that of the NSE group. These observations were confirmed and extended by the measurement of NfL within the Olink Explore panel as shown later in S2 Fig.