Abstract
Chronic liver disease is a major cause of mortality, with approximately 2 million deaths worldwide each year, and it poses a significant economic burden. The most common cause of chronic liver disease in the United States and Europe is steatotic liver disease (SLD), which includes metabolic dysfunction–associated SLD, metabolic dysfunction and alcohol-associated SLD, and alcohol-associated liver disease (ALD). Effective treatment of these conditions is essential to reduce the liver disease burden, with promising approaches including treating cardiometabolic risk factors and excessive alcohol intake. Glucagon-like peptide 1 receptor agonists, both as monotherapy and in combination with other drugs, are gaining attention for their beneficial impact on cardiometabolic risk factors and excessive alcohol intake. In this review, we examine the molecular and clinical effects of glucagon-like peptide 1 receptor agonists, focusing on their direct hepatic steatohepatitis and liver fibrosis but also the indirect influence on cardiometabolic risk factors and excessive alcohol intake as key features of SLD. We also explore the future implications of glucagon-like peptide 1 receptor agonists for treating metabolic dysfunction–associated SLD, metabolic dysfunction and alcohol-associated SLD, alcohol-associated liver disease, and the potential challenges.
Keywords: alcohol-associated liver disease, GLP-1 receptor agonists, metabolic dysfunction–associated steatotic liver disease, nonalcoholic steatohepatitis, treatment
INTRODUCTION
Chronic liver disease is a leading cause of mortality, accounting for approximately 2 million annual deaths worldwide,1,2 and imposes a considerable economic burden with estimated annual financial costs of around $32.5 billion (95% CI: $27.0–$40.4 billion).1 The most common cause of chronic liver disease in the United States and Europe is steatotic liver disease (SLD), including the subcategories metabolic dysfunction–associated steatotic liver disease (MASLD), metabolic dysfunction and alcohol-associated SLD, and alcohol-associated liver disease (ALD).2,3,4 Within the spectrum of SLD, MASLD is the most prevalent type, while metabolic dysfunction and alcohol-associated SLD and ALD are associated with a higher risk of developing severe liver disease and liver-related mortality.5,6
Effective treatments of these conditions are considered the key to reduce the burden of liver disease.7 Treating cardiometabolic risk factors and excessive alcohol intake holds promise for future SLD management. Glucagon-like peptide 1 (GLP-1) receptor agonists as monotherapy and in combination with other drugs are gaining attention due to their beneficial impact on these risk factors.2,8,9
In this review, we discuss the molecular and clinical effects of GLP-1 receptor agonists, with a particular focus on their direct and indirect influence on the key features related to SLD. We also explore the future implications of GLP-1 receptor agonists for the treatment of the specific subclasses of SLD and the potential challenges.
The molecular effects of GLP-1
GLP-1 is a gut hormone and member of the incretin hormone family, secreted by L-cells in the intestinal epithelium in response to the intake of nutrients, especially fats and carbohydrates.10 GLP-1 receptor agonists are synthetic analogs designed to mimic the actions of GLP-1. They have demonstrated beneficial effects on various conditions associated with cardiometabolic diseases.11,12
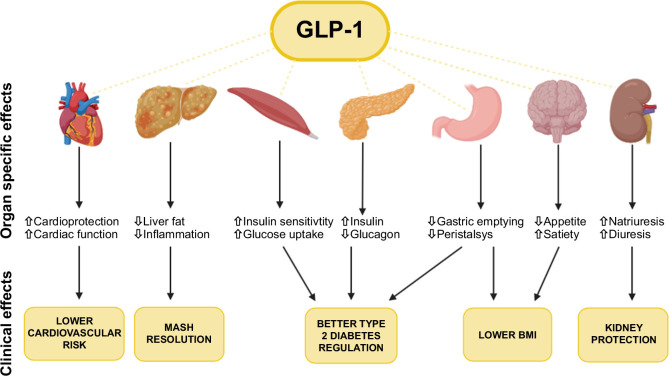
The mode of action is complex as GLP-1 receptor agonists work through multiple pathways in several organ systems (Figure 1).
FIGURE 1.
Illustration of the molecular and clinical effects of GLP-1 receptor agonists. In the uppermost row, a depiction of organs and structures impacted by GLP-1 receptor agonists is presented. Each of these organs exhibits distinct molecular effects. This term covers the outcomes arising either directly or indirectly from the influence of GLP-1 receptor agonists on these organs. In the lower section in the yellow boxes, the clinical effects are described and from what organ they are influenced. Abbreviations: BMI, body mass index; GLP-1, glucagon-like peptide 1; MASH, metabolic dysfunction–associated steatohepatitis.
In the pancreas, GLP-1 receptor agonists bind to receptors on beta cells. This binding increases cAMP levels, which stimulates the release of insulin into the circulation.13,14,15 The increased insulin facilitates glucose transportation into cells, thereby lowering blood glucose levels.15 Additionally, GLP-1 receptor agonists bind to receptors on alpha cells, inhibiting glucagon. This further contributes to lower blood glucose levels by reducing hepatic glucose release.16
In the skeletal muscles, the presence of GLP-1 receptors is still debated.17 Some studies suggest that GLP-1 may increase blood flow in the skeletal muscles and help lower blood glucose levels. However, the precise mechanisms underlying these effects remain unclear.18,19,20
In the stomach, GLP-1 receptor agonists bind to receptors on myenteric neurons in the gastrointestinal (GI) tract, slowing gastric emptying and reducing GI motility.21 This reduced stomach emptying rate decreases the postprandial rise in blood glucose levels and prolongs the feeling of fullness, likely contributing to reduced caloric intake and weight loss.22,23
In the brain, GLP-1 receptor agonists seem to bind to receptors in the hypothalamus, cerebral cortex, and other brain areas.17 This stimulates receptors in the hypothalamus’ satiety centers, reducing hunger perception, which likely contributes to the weight loss effect.24 In the kidneys, GLP-1 receptor agonists inhibit the renal sodium-hydrogen exchanger in proximal tubular cells, increasing diuresis through increased sodium excretion. Additionally, GLP-1 receptor agonists have demonstrated a reduction in renal inflammation.25,26,27
In the liver, there is still ongoing debate regarding the presence and functional significance of GLP-1 receptors.28,29,30 Therefore, the potential beneficial effect on SLD appears to be mediated by treating cardiometabolic risk factors and reducing alcohol intake.
GLP-1 receptor agonists
The discovery of the GLP-1 receptor led to the development of GLP-1 receptor agonists. Since the first formulation was tested, several analogs have been developed to optimize their effects, administration routes, and half-lives in order to enhance compliance.31,32 These analogs exhibit different pharmacokinetic and pharmacodynamic characteristics.33 (Table 1) Their effect varies among the different analogs in terms of weight loss impact and improvements in hemoglobin A1c and cholesterol levels.34 The pathogenesis of metabolic-associated steatohepatitis (MASH) includes insulin resistance in both liver and adipose tissue, which contributes to extra lipid accumulation in the liver and lipotoxicity, leading to liver fibrosis.35,36 Therefore, treatment with GLP-1 receptor agonists that reduce lipid accumulation and insulin resistance may have the potential for a disease-modifying role in MASH.35
TABLE 1.
Most used GLP1-receptor agonists: Descriptions of the different GLP-1 receptor agonists
| Generic name | Active component | Administration | Half-time | Approved indications |
|---|---|---|---|---|
| GLP-1 receptor agonists | ||||
| Exenatide | GLP-1 | SC | 2.4 h | T2DM |
| Lixisenatide | GLP-1 | SC | 3 h | T2DM |
| Liraglutide | GLP-1 | SC | 13 h | T2DM Obesity |
| Semaglutide | GLP-1 | SC + PO | 1 wk | T2DM Obesity |
| Dulaglutide | GLP-1 | SC | 5 d | T2DM |
| Dual agonists | ||||
| Tirzepatide | GLP-1 and glucose-dependent insulinotropic polypeptide | SC | 5 d | T2DM Obesity |
| Survodutide | GLP-1 and glucagon | SC | 6 d | Not yet approved |
| Efinopegdutide | GLP-1 and glucagon | SC | 8 d | Not yet approved |
Abbreviations: PO, per oral; T2DM, type 2 diabetes mellitus.
In recent years, there has been a development in treatments with additional hormone receptor agonists in combination with GLP-1 receptor agonists (dual and triple hormone agonists).37,38,39 These include components such as glucose-dependent insulinotropic polypeptide and glucagon, and combinations have been shown to increase the effect of weight loss.37,39,40 The dual agonists seem to have an enhancing effect, and combining them can lead to synergistic effects and impact hepatocyte metabolism.8,41,42
GLP-1 receptor agonists for treating risk factors for progressive SLD
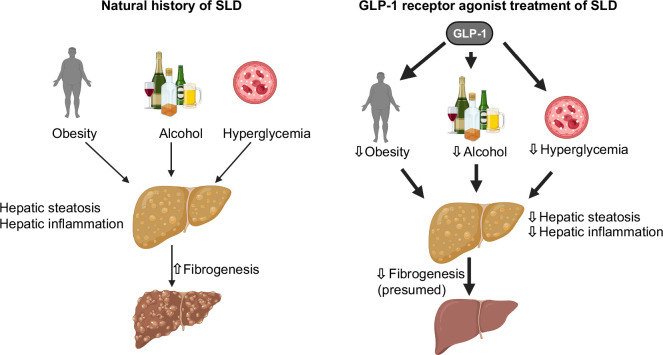
The main risk factors for SLD are cardiometabolic factors, particularly obesity and type 2 diabetes, as well as alcohol intake. However, since GLP-1 receptor agonists have beneficial effects on these risk factors found in most patients with SLD, they are likely to have a positive impact on the progression of SLD (Figure 2). Consequently, for most patients without advanced liver fibrosis, the main benefits of GLP-1 receptor agonists are related to their extrahepatic effects and improvement in cardiometabolic health.43 In contrast, patients with SLD and advanced fibrosis have a relatively high risk of developing decompensated cirrhosis and related complications, which may be reduced or prevented with GLP-1 receptor agonist treatment.43,44
FIGURE 2.
Illustration of the process from steatotic liver disease to a healthy liver using GLP-1 receptor agonists: GLP-1 receptor agonists inhibit risk factors (insulin resistance and excessive alcohol, obesity), promoting reduced hepatic steatosis and inflammation. Abbreviations: GLP-1, glucagon-like peptide 1; SLD, steatotic liver disease.
Managing type 2 diabetes
Most people with type 2 diabetes (T2D) have hepatic steatosis, and T2D is a major risk factor for developing hepatic inflammation (metabolic-associated steatohepatitis (MASH), leading to fibrosis and progression toward cirrhosis.45,46 Dietetic and drug-induced improvements in glycemic control lead to reduced hepatic inflammation.46,47,48,49 Therefore, GLP-1 receptor agonists should be considered in patients with T2D and steatosis.
Managing obesity
Increasing body mass index is directly associated with increasing risk of liver-related morbidity and mortality in SLD.50,51,52 Adipose tissues are one of the main etiologies in developing SLD.53 Excessive fat accumulation in the body can extend to the liver, causing dysregulation. This dysregulation leads to the development of SLD, MASH, and later fibrosis.54 To achieve fibrosis regression, studies with paired biopsies have found that a 7%–10% loss of body weight is required.53
In the European Association for the Study of the Liver Clinical Practice Guidelines on the management of MASLD, incretin-based weight loss drugs are recommended to be considered in patients with MASLD and overweight or obesity.55
Two studies, including 1569 participants, tested semaglutide in people with overweight or obesity, and overall weight loss was obtained. However, the studies also concluded that the weight loss was not maintained after discontinuation.56,57 Thus, sustained weight loss is crucial for reducing liver-related morbidity and mortality in SLD.
Managing excessive alcohol use
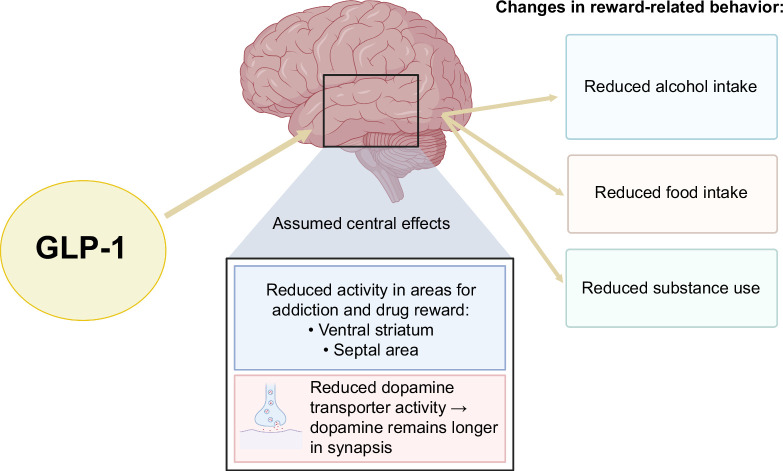
Alcohol is a risk factor for the progression of liver disease in patients with alcohol-associated liver disease (ALD) but also in patients with type 2 diabetes mellitus (T2DM).52,58,59 Vice versa, alcohol abstinence improves the prognosis of patients with cirrhosis and increases hepatic regeneration (regression of liver fibrosis).60,61,62 Therefore, treatments with medications that reduce alcohol consumption are likely to be beneficial for patients with SLD. The neurobiological foundations of addictive disorders, including alcohol use disorder, have led to investigations into the potential use of GLP-1 receptor agonists in addictive disorders.63 This has led to a series of studies primarily conducted in laboratory animals injected with GLP-1 receptor agonists, which have shown a reduction in alcohol intake in rats and mice.63,64 Figure 3 illustrates the proposed central mechanisms of GLP-1 receptor agonists in reducing reward-related behavior, highlighting their potential to drive clinical improvements in treating excessive alcohol consumption, eating disorders, and substance use disorders.
FIGURE 3.
GLP-1 is assumed to influence brain regions linked to reward and addiction, reducing activity in the ventral striatum and septal area. It also decreases the activity of the dopamine transporter, which leads to prolonged dopamine presence in the synapse. This causes a presumed effect in reward-related behavior.65,66 Abbreviation: GLP-1, glucagon-like peptide 1.
One randomized controlled trial testing the GLP-1 receptor agonist exenatide on people with alcohol use disorder found no overall significant reduction in the quantity of alcohol intake or heavy drinking days.65 However, in the subgroup of individuals with obesity, both the quantity of alcohol intake and heavy drinking days were significantly reduced. Neurobiological activity was observed within brain regions associated with drug reward and addiction.65
Another study used self-reported data from individuals taking semaglutide and found that 21% stopped drinking completely, and 88.4% reported a reduced desire for alcohol.67
A retrospective cohort study of over 83,000 electronic health records from patients with obesity found that semaglutide was associated with a 50%–56% lower risk of developing or recurring alcohol use disorder within a 12-month follow-up period compared to other antiobesity drugs.68
Despite the clear association between alcohol intake and the risk of progression of SLD and the above studies suggesting that GLP-1 receptor agonists can reduce alcohol intake, it remains unclear whether treatment with GLP-1 receptor agonists can reduce hepatic inflammation and prevent the progression of fibrosis in patients with SLD.
Preventing extrahepatic morbidity and mortality in patients with SLD
Cardiometabolic-associated diseases are the major competing causes of morbidity and mortality among the most common subclasses of SLD, including MASLD and ALD.43,69,70 Large randomized controlled trials have recently established that GLP-1 receptor agonists can reduce the morbidity and mortality in individuals with overweight and/or T2DM: a randomized trial with 17,604 patients showed that the GLP-1 receptor agonist semaglutide significantly reduced the risk of cardiovascular death with an HR on 0.80 (95% CI: 0.72–0.90).71 Obstructive sleep apnea is associated with major cardiovascular complications. A phase 3 study testing tirzepatide in patients with apnea and obesity resulted in reduced apnea and hypopnea episodes, body weight, systolic blood pressure, and improved sleep-related reports by the patients.72
Another common cause of extrahepatic disease in patients with MASLD is impaired kidney function.43 GLP-1 receptor agonists can have a nephroprotective action in patients with T2DM.73 Results from a study testing GLP-1 receptor agonists on individuals with T2DM stated that GLP-1 receptor agonists obtained kidney-protective effects, including lowering albuminuria and a declining estimated glomerular filtration rate slope.74 The FLOW study published in 2024 testing semaglutide in patients with type 2 diabetes and chronic kidney disease showed a reduced risk of clinically important kidney outcomes and death from cardiovascular causes.75 Given the shared metabolic comorbidities, there is no reason to believe that these beneficial effects would not be seen in patients with SLD.
GLP-1 receptor agonists on SLD
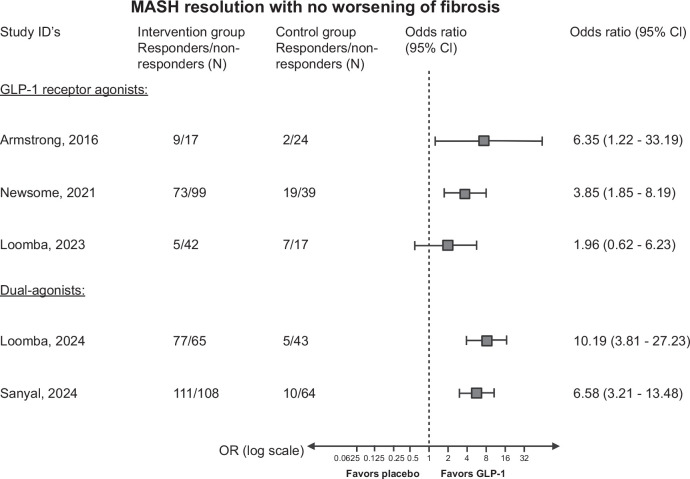
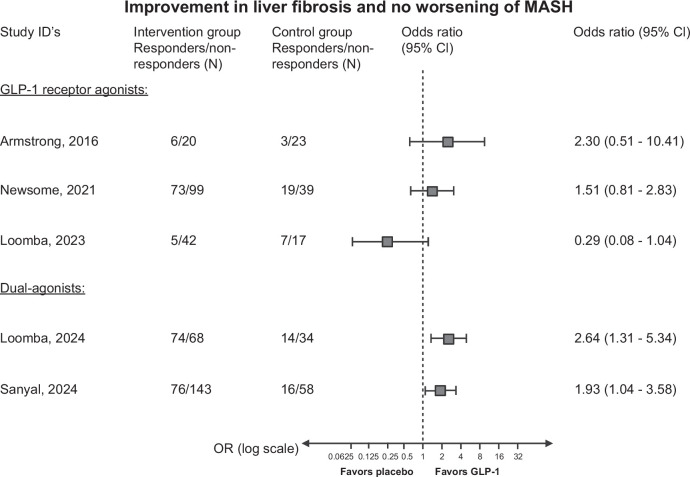
Currently, 5 phase II trials with liver histology have been reported investigating GLP-1 receptor agonists in patients with MASH (Table 2).41,42,76,77,78 Hereof, 3 trials have tested GLP-1 receptor agonists as monotherapy, while 2 studies have tested GLP-1 receptor agonists in combination with glucagon and glucose-dependent insulinotropic polypeptide. The efficacy of MASH resolution and fibrosis regression are summarized in Figures 4 and 5. While there overall seems to be a beneficial effect of GLP-1 receptor agonists, the current evidence suggests that the effect may vary depending on the severity of liver fibrosis, as described below.
TABLE 2.
Clinical trials concerning GLP-1 receptor agonists and liver disease
| References | Agent | Phase | Size/duration | Primary endpoint | Inflammation | Fibrosis |
|---|---|---|---|---|---|---|
| GLP-1 receptor agonists | ||||||
| Armstrong et al76 | Liraglutide, daily sc. | 2 | 52 patients, 48 wk | Resolution of MASH with no worsening of liver fibrosis | MASH resolution with no worsening of fibrosis: 39% in liraglutide group 9% in placebo group p=0.019 |
Improvement in fibrosis stage with no worsening in MASH: 26% in liraglutide 14% in placebo p=0.46 |
| Newsome et al77 | Semaglutide, daily sc. | 2 | 320 patients, 72 wk | Resolution of MASH with no worsening of liver fibrosis | MASH resolution with no worsening of liver fibrosis: 40% in 0.1 mg group 36% in 0.2 mg group 59% in 0.4 mg group 17% in placebo group p<0.001 |
Improvement in fibrosis stage with no worsening in MASH: 43% in 0.4 mg group 33% in placebo group p=0.48 |
| Loomba et al78 | Semaglutide, weekly sc. | 2 | 71 patients, 48 wk | Improvement in liver fibrosis without worsening of MASH | Resolution of MASH: 34% in semaglutide group 21% in placebo group p=0.29 |
Improvement in fibrosis stage with no worsening of MASH: 11% in semaglutide group 29% in placebo group p=0.087 |
| Ongoing phase 3 clinical trials concerning GLP-1 receptor agonists and liver disease | ||||||
| ESSENCE (NCT04822181) | Semaglutide, weekly sc. | 3 | 1200 | Resolution of steatohepatitis and no worsening of liver fibrosis. Improvement in liver fibrosis and no worsening of steatohepatitis. Time to first liver-related event |
Recruiting | Recruiting |
| Dual agonists | ||||||
| Loomba et al41 | Tirzepatide, weekly sc. | 2 | 190 | Resolution of MASH without worsening of fibrosis | MASH resolution with no worsening of fibrosis: 44% in 5 mg group 56% in 10 mg group 62% in 15 mg group 10% in placebo group p<0.001 |
Improvement of ≥1 fibrosis stage with no worsening of MASH; 55% in 5 mg group 51% in 10 mg group 51% in 15 mg group 30% in placebo group |
| Sanyal et al42 | Survodutide, weekly sc. | 2 | 293 | Improvement in MASH with no worsening of fibrosis | Improvement in MASH with no worsening of fibrosis: 47% in 2.4 mg group 62% in 4.8 mg group 43% in 6.0 mg group 14% in placebo group |
Improvement in fibrosis with no worsening of MASH: 34% in 2.4 mg group 36% in 4.8 mg group 34% in 6.0 mg group 22% in placebo group |
Note: We searched Medline for full papers published in any language in peer-reviewed journals up to January 3, 2024. We added the terms “GLP-1” and “liver disease” and filtered by “clinical trial” and identified 58 papers. These papers were manually reviewed and included if their primary endpoint was related to GLP-1 receptor agonists and SLD, used biopsies and were RCTs. We identified 3 papers reporting results of randomized controlled trials of GLP-1 receptor agonists in patients with SLD.
For phase 3 clinical trials, we searched clinicaltrials.gov and clinicaltrialsregister.eu for currently ongoing trials. We added the terms “GLP-1” and “liver disease.” We included the ongoing trials if their primary endpoint was related to GLP-1 receptor agonists and SLD and used biopsies. We identified 1 ongoing phase 3 trials using GLP-1 receptor agonists in patients with SLD.
Abbreviations: GLP-1, glucagon-like peptide 1; MASH, metabolic dysfunction–associated steatohepatitis.
FIGURE 4.
Forest plot with the reported ORs (intention to treat) of MASH resolution and no worsening of fibrosis in the 5 randomized controlled trials on GLP-1 receptor agonists for MASH. It is important to note that the study populations differ regarding fibrosis stage. All data from the randomization until last study-related procedure were included. Missing outcomes were reported as nonresponse. In the study by Newsome et al, only patients with primary outcomes are included in the analysis. Study references (the severity of fibrosis), type of GLP-1 receptor agonist: Armstrong et al76 (F0–F4): liraglutide; Newsome et al77 (F1–F3), Semaglutide, Loomba et al78 (F4): semaglutide, Loomba et al41 (F2–F3): tirzepatide, Sanyal et al42 (F1–F3): survodutide. Abbreviations: GLP-1, glucagon-like peptide 1; MASH, metabolic dysfunction–associated steatohepatitis.
FIGURE 5.
Forest plot with the reported odds ratios (intention to treat) of improvement in liver fibrosis and MASH resolution in the 5 randomized controlled trials on GLP-1 receptor agonists for MASH. It is important to note that the study populations differ regarding fibrosis stage. All data from the randomization until last study-related procedure were included. Missing outcomes were reported as nonresponse. In the study by Newsome et al, only patients with primary outcome are included in the analysis. Study references (the severity of fibrosis), type of GLP-1 receptor agonist: Armstrong et al76 (F0–F4): liraglutide; Newsome et al77 (F1–F3), semaglutide, Loomba et al78 (F4): semaglutide, Loomba et al41 (F2–F3): tirzepatide, Sanyal et al42 (F1–F3): survodutide. Abbreviations: GLP-1, glucagon-like peptide 1; MASH, metabolic dysfunction–associated steatohepatitis.
The first trial investigated the effects of GLP-1 receptor agonist on MASH by testing daily subcutaneous 1.8 mg liraglutide versus placebo for 48 weeks.76 The trial showed that liraglutide induced histological resolution of MASH without worsening liver fibrosis. Additionally, liraglutide seemed to improve the fibrosis stage even though not reaching statistical significance. The second trial tested daily injections of semaglutide in varying doses versus placebo for 72 weeks on patients with biopsy-confirmed MASH and fibrosis stage F1–F3.77 Three doses were tested: 0.1, 0.2, and 0.4 mg. The trial showed that patients receiving the highest dose of 0.4 mg achieved a significant resolution of MASH with no worsening of fibrosis compared to placebo. In contrast to the first trial, semaglutide did not show effect on fibrosis. The third trial tested 2.4 mg semaglutide versus placebo for 48 weeks on patients with biopsy-confirmed F4 (MASH cirrhosis).78 This trial found no difference between the groups on the primary outcome improvement in fibrosis without worsening of MASH. However, there was a significant difference in weight loss and glycemia between the groups.
One phase III trial is currently registered (clinical trial reg. no. NCT04822181, ClinicalTrials.gov). In this trial, semaglutide is being tested against placebo in patients with MASH and fibrosis stages 2 and 3. It is planned to include 1200 participants who will receive treatment for 240 weeks (4.6 y). The primary outcomes are divided into 2 parts.79 The outcome for part 1, as defined in phase II studies, includes “resolution of steatohepatitis with no worsening of liver fibrosis” and “improvement in liver fibrosis with no worsening of steatohepatitis” after 72 weeks (1.4 y). The outcome for part 2 is defined as “cirrhosis-free survival” after 240 weeks (4.6 y) of treatment. The results from part 1 are expected to be presented in late 2024.
A phase 2 trial investigated the effects of weekly injections of survodutide, a dual glucagon and GLP-1 receptor agonist, versus placebo in patients with biopsy-confirmed MASH and fibrosis stages F1–F3 over 48 weeks.42 Survodutide significantly improved MASH without worsening fibrosis compared to placebo. It also reduced liver fat content and showed some improvements in fibrosis stages.
Another dual-agonist tirzepatide consisting of glucose-dependent insulinotropic polypeptide and GLP-1 receptor agonists was also tested in a phase 2 trial in patients with MASH and fibrosis stage F2 or F3.41 Patients received weekly tirzepatide for 52 weeks and showed significant effectiveness over placebo in resolving MASH without worsening of fibrosis. Over 50% in the tirzepatide group improved in the fibrosis stage compared to 30% in the placebo group.
In addition to the clinical trials with predefined purposes to investigate the clinical effects of GLP-1 receptor agonists on MASH with fibrosis, several clinical studies have shown that GLP-1 receptor agonists have beneficial effects on liver enzymes in patients with T2D and patients with overweight.80,81,82 While the impact of GLP-1 receptor agonists on fibrosis remains uncertain, 2 observational studies found a reduced risk of liver-related morbidity in patients with T2D. The first study of 16,659 individuals with chronic liver disease and type 2 diabetes showed that individuals started on GLP-1 receptor agonists had a lower risk of having major adverse liver outcomes.83 The second study, a retrospective study including 1,890,020 patients with T2D, showed that those taking GLP-1 receptor agonists had a reduced risk of incident HCC and hepatic decompensation compared with those taking other types of antidiabetes medication.84
Safety and side effects of GLP-1 receptor agonists
Treatment with GLP-1 receptor agonists is generally well tolerated and the adverse effects that lead to people discontinuing the treatment are mostly GI-related with symptoms, such as nausea, vomiting, and diarrhea.71,85,86 In clinical trials, GI symptoms are estimated to occur in approximately 80% of patients, leading to discontinuation in about 3%–10% of cases.71,75,76,78,87
In larger-scale studies, the incidence of serious adverse events tends to be lower among patients assigned to GLP-1 receptor agonists. This is primarily due to a reduced risk of cardiovascular diseases and serious infections.71,75
In one of the largest trials, testing semaglutide with 17,000 participants, pancreatitis was not significantly more prevalent compared to the placebo group.71 Most data, however, suggest that GLP-1 receptor agonists do not significantly increase the risk of pancreatitis and pancreatic cancer.88,89,90,91,92 In contrast, gallbladder problems, including cholecystitis, cholelithiasis, and biliary obstruction, have been more frequently observed with the use of GLP-1 receptor agonists, and the risks appear to be more pronounced with higher doses and prolonged use of the medications.93,94
In children and adolescents, large-scale evidence regarding the safety of GLP-1 receptor agonists is lacking. However, 2 randomized controlled trials involving 201 adolescents with obesity and 134 children and adolescents with T2D found that adverse effects were very similar to those seen in adults.95,96 The most common adverse events were GI disorders, including nausea. Cholelithiasis was found in 4% of the semaglutide group had cholelithiasis with no cases in the placebo group.96 These safety results are supported by a meta-analysis with similar findings.97
Safety data on GLP-1 receptor agonists in elderly individuals, particularly those over 75 years old, is limited. This is mainly because people over 75 are often excluded from clinical trials. However, the existing data for individuals over 75 does not suggest that the safety profile differs from that of adults under 75.98
Consideration of GLP1-receptor agonists in the treatment of decompensated cirrhosis
While many patients with SLD are likely to benefit from GLP-1 receptor agonists, caution should be exercised in patients with decompensated cirrhosis. Malnutrition is a common condition in patients with decompensated cirrhosis, worsening with disease severity and associated with increasing risk of complications and higher mortality.99 Given the weight loss effect of semaglutide, its benefits and risks should be carefully considered when treating this patient group. Additionally, the likelihood of a beneficial effect appears lower among patients with cirrhosis, as the only study in this patient group to date has not shown any clear beneficial effects (Figures 4 and 5).78 Further, the impact of lean body mass associated with GLP-1 receptor agonists is a concern in patients with decompensated cirrhosis that may already suffer from sarcopenia.100
Real-world evidence
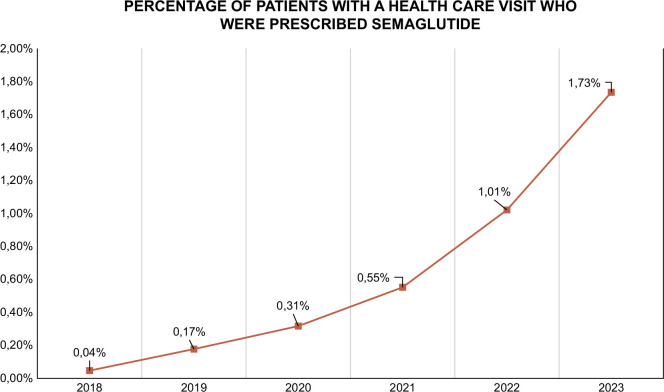
The use of GLP-1 receptor agonists in both type 2 diabetes and obesity has rapidly increased in the last few years, and in the United States, the percentage of patients with a health care visit who were prescribed semaglutide increased from 0.04% in 2018 to 1.73% in 2023 (Figure 6).
FIGURE 6.
Graph of the development in semaglutide prescriptions. Pictured as the percentage of patients with a health care visit who were prescribed semaglutide. The data come from Cosmos, a HIPAA-limited data set of >217 million patients from 218 Epic organizations, including over 1200 hospitals and over 26,000 clinics, serving patients in all 50 states and 3 in Lebanon. The data are collected from January 1, 2018, to September 15, 2023.
While the weight loss effects have significantly increased interest and usage, a paradoxical new phenomenon has emerged. Despite achieving the desired effects on glycemic control and weight loss, a substantial proportion of patients initiating GLP-1 receptor agonist treatment discontinue it.85 A retrospective study with 4791 participants with type 2 diabetes, 48% discontinued the treatment after 12 months and 70% for 24 months.85 Other studies investigating the reasons for discontinuation found that the most common reasons were GI side effects, including nausea and vomiting.71,101 Problems with injections, inadequate blood glucose control, and hypoglycemia were also factors.101 Finally, the financial aspect due to the high cost of GLP-1 receptor agonists also plays a role, with 27% reporting that the medicine was too costly and 13% reporting this as the reason for discontinuation.101
Even though discontinuation of GLP-1 receptor agonists often leads to weight gain and return to baseline of cardiometabolic variables, cardiometabolic improvement can be maintained through supervised exercise, as indicated by a 2024 Danish post-treatment study.102,103
No direct effect on fibrosis, but still hope for future treatment
While cardiometabolic disease, alcohol intake, and genetics are considered the main drivers of progressive SLD, liver fibrosis is the key predictor for the development of cirrhosis and liver-related mortality.44 However, the evidence of GLP-1 receptors being expressed in the liver is debatable, and no trials yet have shown direct effect on liver fibrosis. Therefore, the potential beneficial effect of GLP-1 receptor agonists on liver fibrosis is considered to be driven mainly by weight loss, glycemic control, and lower alcohol intake that lead to indirect beneficial effects on hepatic steatosis and inflammation and, over longer time, fibrosis regression.8 Such a relationship has been observed in bariatric intervention for MASH.104,105 In a study with a 1-year follow-up, hepatic inflammation was affected and had an impact on liver fibrosis.104 Another study with a 5-year follow-up showed fibrosis regression in patients who underwent bariatric intervention.105 This supports the hypothesis that reducing hepatic inflammation can lead to fibrosis regression over time. None of the GLP-1 receptor agonist trials on individuals with MASH have this long follow-up durations, which may explain why no significant effect on fibrosis has been seen. It is noteworthy that the food and drug administration and European Medicines Agency only accept fibrosis reduction or regression as endpoints, excluding stable disease as a validated outcome.106 This means that for a drug to gain approval, it must demonstrate an ability to reduce fibrosis rather than merely halting fibrosis progression, despite having lower stages of liver fibrosis without progression likely affecting the quality of life and having limited clinical consequences.
CONCLUSIONS
The impact of GLP-1 receptor agonists on glycemic control, weight loss, and potentially reducing alcohol intake is highly promising. Since GLP-1 receptor agonists are already used to treat T2D and obesity, and because cardiometabolic disease is closely linked to SLD, many patients could benefit from this treatment, regardless of SLD severity. However, patients with SLD and advanced fibrosis face a higher risk of decompensated cirrhosis and related complications, which may be preventable with GLP-1 receptor agonist. The focus on developing medications for MASH, along with the growing interest in metabolic dysfunction and alcohol-associated SLD and ALD, GLP-1 receptor agonists-based therapies offer hope for effective treatment options in SLD.
Footnotes
Abbreviations: ALD, alcohol-associated liver disease; GI, gastrointestinal; GLP-1, glucagon-like peptide 1; MASH, metabolic-associated steatohepatitis; MASLD, metabolic dysfunction–associated steatotic liver disease; SLD, steatotic liver disease; T2D, type 2 diabetes; T2DM, type 2 diabetes mellitus.
Contributor Information
Ellen L. Jensen, Email: ellen.lyngbeck.jensen@rsyd.dk.
Mads Israelsen, Email: mads.israelsen@rsyd.dk.
Aleksander Krag, Email: Aleksander.Krag@rsyd.dk.
AUTHOR CONTRIBUTIONS
All authors contributed equally to both the writing and critical review of various sections of the manuscript, and all have given their approval for the final version to be submitted.
During the preparation of this work, the authors used ChatGTP to improve language and readability. After using this tool/service, the authors reviewed and edited the content as needed and take full responsibility for the content of the publication.
CONFLICTS OF INTEREST
Mads Israelsen has received travel support from Novo Nordisk in relation to the “7730 ALD” investigator meeting. Aleksander Krag has served as speaker for Novo Nordisk, Norgine, and Siemens and participated in advisory boards for Norgine, Siemens, Boehringer Ingelheim, and Novo Nordisk, all outside the submitted work. Research support: Norgine, Siemens, Nordic Bioscience, AstraZeneca, Echosens. Consulting: Takeda, Resalis Therapeutics, and Zealand Pharma. Board member and co-founder Evido. Ellen L. Jensen has no conflicts to report.
REFERENCES
- 1.Devarbhavi H, Asrani SK, Arab JP, Nartey YA, Pose E, Kamath PS. Global burden of liver disease: 2023 update. J Hepatol. 2023;79:516–37. [DOI] [PubMed] [Google Scholar]
- 2.Karlsen TH, Sheron N, Zelber-Sagi S, Carrieri P, Dusheiko G, Bugianesi E, et al. The EASL-Lancet Liver Commission: Protecting the next generation of Europeans against liver disease complications and premature mortality. Lancet. 2022;399:61–116. [DOI] [PubMed] [Google Scholar]
- 3.Wong VWS, Ekstedt M, Wong GLH, Hagström H. Changing epidemiology, global trends and implications for outcomes of NAFLD. J Hepatol. 2023;79:842–52. [DOI] [PubMed] [Google Scholar]
- 4.Staufer K, Stauber RE. Steatotic liver disease: Metabolic dysfunction, alcohol, or both. Biomedicines. 2023;11:2108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lee BP, Dodge JL, Terrault NA. National prevalence estimates for steatotic liver disease and subclassifications using consensus nomenclature. Hepatology. 2023;79:666–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Israelsen M, Torp N, Johansen S, Hansen CD, Hansen ED, Thorhauge K, et al. Validation of the new nomenclature of steatotic liver disease in patients with a history of excessive alcohol intake: An analysis of data from a prospective cohort study. Lancet Gastroenterol Hepatol. 2024;9:218–28. [DOI] [PubMed] [Google Scholar]
- 7.Karlsen TH, Rutter H, Carrieri P, Zelber-Sagi S, Engebretsen E, Hutchinson S, et al. The EASL Lancet Commission on liver health in Europe: Prevention, case-finding, and early diagnosis to reduce liver-related mortality. Lancet. 2024;403:1522–24. [DOI] [PubMed] [Google Scholar]
- 8.Newsome PN, Ambery P. Incretins (GLP-1 receptor agonists and dual/triple agonists) and the liver. J Hepatol. 2023;79:1557–65. [DOI] [PubMed] [Google Scholar]
- 9.Israelsen M, Torp N, Johansen S, Thiele M, Krag A. MetALD: New opportunities to understand the role of alcohol in steatotic liver disease. Lancet Gastroenterol Hepatol. 2023;8:866–868. [DOI] [PubMed] [Google Scholar]
- 10.Brown E, Heerspink HJL, Cuthbertson DJ, Wilding JPH. SGLT2 inhibitors and GLP-1 receptor agonists: Established and emerging indications. Lancet. 2021;398:262–76. [DOI] [PubMed] [Google Scholar]
- 11.Samson SL, Vellanki P, Blonde L, Christofides EA, Galindo RJ, Hirsch IB, et al. American Association of Clinical Endocrinology Consensus Statement: Comprehensive type 2 diabetes management algorithm—2023 update. Endocr Pract. 2023;29:305–40. [DOI] [PubMed] [Google Scholar]
- 12.Wang JY, Wang QW, Yang XY, Yang W, Li DR, Jin JY, et al. GLP-1 receptor agonists for the treatment of obesity: Role as a promising approach. Front Endocrinol (Lausanne). 2023;14:1085799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ding W-G, Renström E, Rorsman P, Buschard K, Gromada J. Glucagon-like peptide I and glucose-dependent insulinotropic polypeptide stimulate Ca2+-induced secretion in rat α-cells by a protein kinase A–mediated mechanism. Diabetes. 1997;46:792–800. [DOI] [PubMed] [Google Scholar]
- 14.Chaudhuri A, Ghanim H, Makdissi A, Green K, Abuaysheh S, Batra M, et al. Exenatide induces an increase in vasodilatory and a decrease in vasoconstrictive mediators. Diabetes Obes Metab. 2017;19:729–33. [DOI] [PubMed] [Google Scholar]
- 15.Kapitza C, Dahl K, Jacobsen JB, Axelsen MB, Flint A. Effects of semaglutide on beta cell function and glycaemic control in participants with type 2 diabetes: a randomised, double-blind, placebo-controlled trial. Diabetologia. 2017;60:1390–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang Y, Parajuli KR, Fava GE, Gupta R, Xu W, Nguyen LU, et al. GLP-1 receptor in pancreatic α-cells regulates glucagon secretion in a glucose-dependent bidirectional manner. Diabetes. 2019;68:34–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Müller TD, Finan B, Bloom SR, D'Alessio D, Drucker DJ, Flatt PR, et al. Glucagon-like peptide 1 (GLP-1). Mol Metab. 2019;30:72–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Abdulla H, Phillips B, Wilkinson D, Gates A, Limb M, Jandova T, et al. Effects of GLP-1 infusion upon whole-body glucose uptake and skeletal muscle perfusion during fed-state in older men. J Clin Endocrinol Metab. 2023;108:971–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Green CJ, Henriksen TI, Pedersen BK, Solomon TPJ. Glucagon like peptide-1-induced glucose metabolism in differentiated human muscle satellite cells is attenuated by hyperglycemia. PLoS One. 2012;7:e44284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Asmar A, Asmar M, Simonsen L, Madsbad S, Holst JJ, Hartmann B, et al. Glucagon-like peptide-1 elicits vasodilation in adipose tissue and skeletal muscle in healthy men. Physiol Rep. 2017;5:e13073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Halim MA, Degerblad M, Sundbom M, Karlbom U, Holst JJ, Webb DL, et al. Glucagon-like peptide-1 inhibits prandial gastrointestinal motility through myenteric neuronal mechanisms in humans. J Clin Endocrinol Metab. 2018;103:575–85. [DOI] [PubMed] [Google Scholar]
- 22.Drucker DJ. Mechanisms of action and therapeutic application of glucagon-like peptide-1. Cell Metab. 2018;27:740–56. [DOI] [PubMed] [Google Scholar]
- 23.van Can J, Sloth B, Jensen CB, Flint A, Blaak EE, Saris WHM. Effects of the once-daily GLP-1 analog liraglutide on gastric emptying, glycemic parameters, appetite and energy metabolism in obese, non-diabetic adults. Int J Obes (Lond). 2014;38:784–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Holt MK, Richards JE, Cook DR, Brierley DI, Williams DL, Reimann F, et al. Preproglucagon neurons in the nucleus of the solitary tract are the main source of brain GLP-1, mediate stress-induced hypophagia, and limit unusually large intakes of food. Diabetes. 2019;68:21–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tonneijck L, Muskiet MHA, Blijdorp CJ, Smits MM, Twisk JW, Kramer MHH, et al. Renal tubular effects of prolonged therapy with the GLP-1 receptor agonist lixisenatide in patients with type 2 diabetes mellitus. Am J Physiol Renal Physiol. 2019;316:F231–40. [DOI] [PubMed] [Google Scholar]
- 26.Gutzwiller JP, Tschopp S, Bock A, Zehnder CE, Huber AR, Kreyenbuehl M, et al. Glucagon-like peptide 1 induces natriuresis in healthy subjects and in insulin-resistant obese men. J Clin Endocrinol Metab. 2004;89:3055–61. [DOI] [PubMed] [Google Scholar]
- 27.Lee YS, Jun HS. Anti-inflammatory effects of GLP-1-based therapies beyond glucose control. Mediators Inflamm. 2016;2016:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bullock BP, Heller RS, Habener JF. Tissue distribution of messenger ribonucleic acid encoding the rat glucagon-like peptide-1 receptor. Endocrinology. 1996;137:2968–78. [DOI] [PubMed] [Google Scholar]
- 29.Gupta NA, Mells J, Dunham RM, Grakoui A, Handy J, Saxena NK, et al. Glucagon-like peptide-1 receptor is present on human hepatocytes and has a direct role in decreasing hepatic steatosis in vitro by modulating elements of the insulin signaling pathway. Hepatology. 2010;51:1584–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yokomori H, Ando W. Spatial expression of glucagon-like peptide 1 receptor and caveolin-1 in hepatocytes with macrovesicular steatosis in non-alcoholic steatohepatitis. BMJ Open Gastroenterol. 2020;7:e000370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Drucker DJ, Habener JF, Holst JJ. Discovery, characterization, and clinical development of the glucagon-like peptides. J Clin Invest. 2017;127:4217–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.DeFronzo RA, Ratner RE, Han J, Kim DD, Fineman MS, Baron AD. Effects of exenatide (exendin-4) on glycemic control and weight over 30 weeks in metformin-treated patients with type 2 diabetes. Diabetes Care. 2005;28:1092–100. [DOI] [PubMed] [Google Scholar]
- 33.Nauck MA, Quast DR, Wefers J, Meier JJ. GLP-1 receptor agonists in the treatment of type 2 diabetes—State-of-the-art. Mol Metab. 2021;46:101102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yao H, Zhang A, Li D, Wu Y, Wang CZ, Wan JY, et al. Comparative effectiveness of GLP-1 receptor agonists on glycaemic control, body weight, and lipid profile for type 2 diabetes: Systematic review and network meta-analysis. Bmj. 2024;384:e076410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Armstrong MJ, Hull D, Guo K, Barton D, Hazlehurst JM, Gathercole LL, et al. Glucagon-like peptide 1 decreases lipotoxicity in non-alcoholic steatohepatitis. J Hepatol. 2016;64:399–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tacke F, Puengel T, Loomba R, Friedman SL. An integrated view of anti-inflammatory and antifibrotic targets for the treatment of NASH. J Hepatol. 2023;79:552–66. [DOI] [PubMed] [Google Scholar]
- 37.Jastreboff AM, Aronne LJ, Ahmad NN, Wharton S, Connery L, Alves B, et al. Tirzepatide once weekly for the treatment of obesity. N Engl J Med. 2022;387:205–16. [DOI] [PubMed] [Google Scholar]
- 38.Rosenstock J, Frias J, Jastreboff AM, Du Y, Lou J, Gurbuz S, et al. Retatrutide, a GIP, GLP-1 and glucagon receptor agonist, for people with type 2 diabetes: A randomised, double-blind, placebo and active-controlled, parallel-group, phase 2 trial conducted in the USA. Lancet. 2023;402:529–44. [DOI] [PubMed] [Google Scholar]
- 39.Jastreboff AM, Kaplan LM, Frías JP, Wu Q, Du Y, Gurbuz S, et al. Triple-hormone-receptor agonist retatrutide for obesity—A phase 2 trial. N Engl J Med. 2023;389:514–26. [DOI] [PubMed] [Google Scholar]
- 40.Frías JP, Davies MJ, Rosenstock J, Pérez Manghi FC, Fernández Landó L, Bergman BK, et al. Tirzepatide versus semaglutide once weekly in patients with type 2 diabetes. N Engl J Med. 2021;385:503–15. [DOI] [PubMed] [Google Scholar]
- 41.Loomba R, Hartman ML, Lawitz EJ, Vuppalanchi R, Boursier J, Bugianesi E, et al. Tirzepatide for metabolic dysfunction-associated steatohepatitis with liver fibrosis. N Engl J Med. 2024;391:299–310. [DOI] [PubMed] [Google Scholar]
- 42.Sanyal AJ, Bedossa P, Fraessdorf M, Neff GW, Lawitz E, Bugianesi E, et al. A phase 2 randomized trial of survodutide in MASH and fibrosis. N Engl J Med. 2024;391:311–9. [DOI] [PubMed] [Google Scholar]
- 43.Sanyal AJ, Van Natta ML, Clark J, Neuschwander-Tetri BA, Diehl A, Dasarathy S, et al. Prospective study of outcomes in adults with nonalcoholic fatty liver disease. N Engl J Med. 2021;385:1559–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Israelsen M, Guerrero Misas M, Koutsoumourakis A, Huang Y, Thiele M, Hall A, et al. Collagen proportionate area predicts clinical outcomes in patients with alcohol-related liver disease. Aliment Pharmacol Ther. 2020;52:1728–39. [DOI] [PubMed] [Google Scholar]
- 45.Lee CH, Lui DT, Lam KS. Non-alcoholic fatty liver disease and type 2 diabetes: An update. J Diabetes Investig. 2022;13:930–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hansen CD, Gram-Kampmann EM, Hansen JK, Hugger MB, Madsen BS, Jensen JM, et al. Effect of calorie-unrestricted low-carbohydrate, high-fat diet versus high-carbohydrate, low-fat diet on type 2 diabetes and nonalcoholic fatty liver disease: A randomized controlled trial. Ann Intern Med. 2023;176:10–21. [DOI] [PubMed] [Google Scholar]
- 47.Davies M, Pieber TR, Hartoft-Nielsen ML, Hansen OKH, Jabbour S, Rosenstock J. Effect of oral semaglutide compared with placebo and subcutaneous semaglutide on glycemic control in patients with type 2 diabetes: A randomized clinical trial. JAMA. 2017;318:1460–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nauck MA, Petrie JR, Sesti G, Mannucci E, Courrèges JP, Lindegaard ML, et al. A phase 2, randomized, dose-finding study of the novel once-weekly human GLP-1 analog, semaglutide, compared with placebo and open-label liraglutide in patients with type 2 diabetes. Diabetes Care. 2016;39:231–41. [DOI] [PubMed] [Google Scholar]
- 49.Shi Q, Nong K, Vandvik PO, Guyatt GH, Schnell O, Rydén L, et al. Benefits and harms of drug treatment for type 2 diabetes: Systematic review and network meta-analysis of randomised controlled trials. BMJ. 2023;381:e074068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Israelsen M, Juel HB, Detlefsen S, Madsen BS, Rasmussen DN, Larsen TR, et al. Metabolic and genetic risk factors are the strongest predictors of severity of alcohol-related liver fibrosis. Clin Gastroenterol Hepatol. 2022;20:1784–94.e9. [DOI] [PubMed] [Google Scholar]
- 51.Sahlman P, Nissinen M, Puukka P, Jula A, Salomaa V, Männistö S, et al. Genetic and lifestyle risk factors for advanced liver disease among men and women. J Gastroenterol Hepatol. 2020;35:291–8. [DOI] [PubMed] [Google Scholar]
- 52.Åberg F, Luukkonen PK, But A, Salomaa V, Britton A, Petersen KM, et al. Development and validation of a model to predict incident chronic liver disease in the general population: The CLivD score. J Hepatol. 2022;77:302–11. [DOI] [PubMed] [Google Scholar]
- 53.Glass O, Filozof C, Noureddin M, Berner-Hansen M, Schabel E, Omokaro SO, et al. Standardisation of diet and exercise in clinical trials of NAFLD-NASH: Recommendations from the Liver Forum. J Hepatol. 2020;73:680–93. [DOI] [PubMed] [Google Scholar]
- 54.Lee E, Korf H, Vidal-Puig A. An adipocentric perspective on the development and progression of non-alcoholic fatty liver disease. J Hepatol. 2023;78:1048–62. [DOI] [PubMed] [Google Scholar]
- 55.Tacke F, Horn P, Wai-Sun Wong V, Ratziu V, Bugianesi E, Francque S, et al. EASL-EASD-EASO Clinical Practice Guidelines on the management of metabolic dysfunction-associated steatotic liver disease (MASLD). J Hepatol. 2024;81:492–542. [DOI] [PubMed] [Google Scholar]
- 56.Knop FK, Aroda VR, do Vale RD, Holst-Hansen T, Laursen PN, Rosenstock J, et al. Oral semaglutide 50 mg taken once per day in adults with overweight or obesity (OASIS 1): A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2023;402:705–19. [DOI] [PubMed] [Google Scholar]
- 57.Rubino D, Abrahamsson N, Davies M, Hesse D, Greenway FL, Jensen C, et al. Effect of continued weekly subcutaneous semaglutide vs placebo on weight loss maintenance in adults with overweight or obesity: The STEP 4 randomized clinical trial. JAMA. 2021;325:1414–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rasmussen DN, Thiele M, Johansen S, Kjærgaard M, Lindvig KP, Israelsen M, et al. Prognostic performance of 7 biomarkers compared to liver biopsy in early alcohol-related liver disease. J Hepatol. 2021;75:1017–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mallet V, Parlati L, Martinino A, Scarano Pereira JP, Jimenez CN, Sakka M, et al. Burden of liver disease progression in hospitalized patients with type 2 diabetes mellitus. J Hepatol. 2022;76:265–74. [DOI] [PubMed] [Google Scholar]
- 60.Hofer BS, Simbrunner B, Hartl L, Jachs M, Bauer DJM, Balcar L, et al. Alcohol abstinence improves prognosis across all stages of portal hypertension in alcohol-related cirrhosis. Clin Gastroenterol Hepatol. 2023;21:2308–17.e7. [DOI] [PubMed] [Google Scholar]
- 61.Louvet A, Bourcier V, Archambeaud I, d’Alteroche L, Chaffaut C, Oberti F, et al. Low alcohol consumption influences outcomes in individuals with alcohol-related compensated cirrhosis in a French multicenter cohort. J Hepatol. 2023;78:501–12. [DOI] [PubMed] [Google Scholar]
- 62.Israelsen M, Madsen BS, Torp N, Johansen S, Hansen CD, Detlefsen S, et al. Rifaximin-α for liver fibrosis in patients with alcohol-related liver disease (GALA-RIF): A randomised, double-blind, placebo-controlled, phase 2 trial. Lancet Gastroenterol Hepatol. 2023;8:523–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chuong V, Farokhnia M, Khom S, Pince CL, Elvig SK, Vlkolinsky R, et al. The glucagon-like peptide-1 (GLP-1) analogue semaglutide reduces alcohol drinking and modulates central GABA neurotransmission. JCI Insight. 2023;8:e170671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Marty VN, Farokhnia M, Munier JJ, Mulpuri Y, Leggio L, Spigelman I. Long-acting glucagon-like peptide-1 receptor agonists suppress voluntary alcohol intake in male Wistar rats. Front Neurosci. 2020;14:599646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Klausen MK, Jensen ME, Møller M, Le Dous N, Jensen AMØ, Zeeman VA, et al. Exenatide once weekly for alcohol use disorder investigated in a randomized, placebo-controlled clinical trial. JCI Insight. 2022;7. doi: 10.1172/jci.insight.159863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Klausen MK, Thomsen M, Wortwein G, Fink‐Jensen A. The role of glucagon-like peptide 1 (GLP-1) in addictive disorders. Br J Pharmacol. 2022;179:625–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Patton C, Brooks AE, Brooks BD, McKelvey K. Semaglutide: A promising treatment for alcohol use disorder. Preprints: Preprints. 2023. doi: 10.20944/preprints202310.1883.v1 [DOI] [Google Scholar]
- 68.Wang W, Volkow ND, Berger NA, Davis PB, Kaelber DC, Xu R. Associations of semaglutide with incidence and recurrence of alcohol use disorder in real-world population. Nature Commun. 2024;15:4548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kann AE, Jepsen P, Madsen LG, West J, Askgaard G. Cause-specific mortality in patients with alcohol-related liver disease in Denmark: A population-based study. Lancet Gastroenterol Hepatol. 2023;8:1028–34. [DOI] [PubMed] [Google Scholar]
- 70.Simon TG, Roelstraete B, Hagström H, Sundström J, Ludvigsson JF. Non-alcoholic fatty liver disease and incident major adverse cardiovascular events: Results from a nationwide histology cohort. Gut. 2022;71:1867–75. [DOI] [PubMed] [Google Scholar]
- 71.Lincoff AM, Brown-Frandsen K, Colhoun HM, Deanfield J, Emerson SS, Esbjerg S, et al. Semaglutide and cardiovascular outcomes in obesity without diabetes. N Engl J Med. 2023;389:2221–32. [DOI] [PubMed] [Google Scholar]
- 72.Malhotra A, Grunstein RR, Fietze I, Weaver TE, Redline S, Azarbarzin A, et al. Tirzepatide for the treatment of obstructive sleep apnea and obesity. N Engl J Med. 2024. doi: 10.1056/NEJMoa2404881 [DOI] [PubMed] [Google Scholar]
- 73.Granata A, Maccarrone R, Anzaldi M, Leonardi G, Pesce F, Amico F, et al. GLP-1 receptor agonists and renal outcomes in patients with diabetes mellitus type 2 and diabetic kidney disease: State of the art. Clin Kidney J. 2022;15:1657–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Shaman AM, Bain SC, Bakris GL, Buse JB, Idorn T, Mahaffey KW, et al. Effect of the glucagon-like peptide-1 receptor agonists semaglutide and liraglutide on kidney outcomes in patients with type 2 diabetes: Pooled analysis of SUSTAIN 6 and LEADER. Circulation. 2022;145:575–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Perkovic V, Tuttle KR, Rossing P, Mahaffey KW, Mann JFE, Bakris G, et al. Effects of semaglutide on chronic kidney disease in patients with type 2 diabetes. N Engl J Med. 2024;391:109–21. [DOI] [PubMed] [Google Scholar]
- 76.Armstrong MJ, Gaunt P, Aithal GP, Barton D, Hull D, Parker R, et al. Liraglutide safety and efficacy in patients with non-alcoholic steatohepatitis (LEAN): a multicentre, double-blind, randomised, placebo-controlled phase 2 study. Lancet. 2016;387:679–90. [DOI] [PubMed] [Google Scholar]
- 77.Newsome PN, Buchholtz K, Cusi K, Linder M, Okanoue T, Ratziu V, et al. A placebo-controlled trial of subcutaneous semaglutide in nonalcoholic steatohepatitis. N Engl J Med. 2021;384:1113–24. [DOI] [PubMed] [Google Scholar]
- 78.Loomba R, Abdelmalek MF, Armstrong MJ, Jara M, Kjær MS, Krarup N, et al. Semaglutide 2·4 mg once weekly in patients with non-alcoholic steatohepatitis-related cirrhosis: A randomised, placebo-controlled phase 2 trial. Lancet Gastroenterol Hepatol. 2023;8:511–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Noncirrhotic Nonalcoholic Steatohepatitis With Liver Fibrosis: Developing Drugs for Treatment. U.S. Food and Drug Administration (U.S. Food and Drug Administration) 2023.
- 80.Liu L, Yan H, Xia M, Zhao L, Lv M, Zhao N, et al. Efficacy of exenatide and insulin glargine on nonalcoholic fatty liver disease in patients with type 2 diabetes. Diabetes Metab Res Rev. 2020;36:e3292. [DOI] [PubMed] [Google Scholar]
- 81.Davies M, Færch L, Jeppesen OK, Pakseresht A, Pedersen SD, Perreault L, et al. Semaglutide 2·4 mg once a week in adults with overweight or obesity, and type 2 diabetes (STEP 2): A randomised, double-blind, double-dummy, placebo-controlled, phase 3 trial. Lancet. 2021;397:971–84. [DOI] [PubMed] [Google Scholar]
- 82.Flint A, Andersen G, Hockings P, Johansson L, Morsing A, Sundby Palle M, et al. Randomised clinical trial: Semaglutide versus placebo reduced liver steatosis but not liver stiffness in subjects with non-alcoholic fatty liver disease assessed by magnetic resonance imaging. Aliment Pharmacol Ther. 2021;54:1150–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wester A, Shang Y, Toresson Grip E, Matthews AA, Hagström H. Glucagon-like peptide-1 receptor agonists and risk of major adverse liver outcomes in patients with chronic liver disease and type 2 diabetes. Gut. 2024;73:835–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wang L, Berger NA, Kaelber DC, Xu R. Association of GLP-1 receptor agonists and hepatocellular carcinoma incidence and hepatic decompensation in patients with type 2 diabetes. Gastroenterology. 2024;167:689–703. [DOI] [PubMed] [Google Scholar]
- 85.Weiss T, Carr RD, Pal S, Yang L, Sawhney B, Boggs R, et al. Real-world adherence and discontinuation of glucagon-like peptide-1 receptor agonists therapy in type 2 diabetes mellitus patients in the United States. Patient Prefer Adherence. 2020;14:2337–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Shetty R, Basheer FT, Poojari PG, Thunga G, Chandran VP, Acharya LD. Adverse drug reactions of GLP-1 agonists: A systematic review of case reports. Diabetes Metab Syndr. 2022;16:102427. [DOI] [PubMed] [Google Scholar]
- 87.Wadden TA, Bailey TS, Billings LK, Davies M, Frias JP, Koroleva A, et al. Effect of subcutaneous semaglutide vs placebo as an adjunct to intensive behavioral therapy on body weight in adults with overweight or obesity: The STEP 3 randomized clinical trial. JAMA. 2021;325:1403–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Abd El Aziz M, Cahyadi O, Meier JJ, Schmidt WE, Nauck MA. Incretin-based glucose-lowering medications and the risk of acute pancreatitis and malignancies: A meta-analysis based on cardiovascular outcomes trials. Diabetes Obes Metab. 2020;22:699–704. [DOI] [PubMed] [Google Scholar]
- 89.Nauck MA, Frossard JL, Barkin JS, Anglin G, Hensley IE, Harper KD, et al. Assessment of pancreas safety in the development program of once-weekly GLP-1 receptor agonist dulaglutide. Diabetes Care. 2017;40:647–54. [DOI] [PubMed] [Google Scholar]
- 90.Azoulay L, Filion KB, Platt RW, Dahl M, Dormuth CR, Clemens KK, et al. Incretin based drugs and the risk of pancreatic cancer: International multicentre cohort study. BMJ. 2016;352:i581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Azoulay L, Filion KB, Platt RW, Dahl M, Dormuth CR, Clemens KK, et al. Association between incretin-based drugs and the risk of acute pancreatitis. JAMA Intern Med. 2016;176:1464–73. [DOI] [PubMed] [Google Scholar]
- 92.Wilhite K, Reid JM, Lane M. Risk of pancreatitis with incretin therapies versus thiazolidinediones in the Veterans Health Administration. Ann Pharmacother. 2024;58:685–9. [DOI] [PubMed] [Google Scholar]
- 93.He L, Wang J, Ping F, Yang N, Huang J, Li Y, et al. Association of glucagon-like peptide-1 receptor agonist use with risk of gallbladder and biliary diseases: A systematic review and meta-analysis of randomized clinical trials. JAMA Intern Med. 2022;182:513–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Faillie JL, Yu OH, Yin H, Hillaire-Buys D, Barkun A, Azoulay L. Association of bile duct and gallbladder diseases with the use of incretin-based drugs in patients with type 2 diabetes mellitus. JAMA Intern Med. 2016;176:1474–81. [DOI] [PubMed] [Google Scholar]
- 95.Tamborlane WV, Barrientos-Pérez M, Fainberg U, Frimer-Larsen H, Hafez M, Hale PM, et al. Liraglutide in children and adolescents with type 2 diabetes. N Engl J Med. 2019;381:637–46. [DOI] [PubMed] [Google Scholar]
- 96.Weghuber D, Barrett T, Barrientos-Pérez M, Gies I, Hesse D, Jeppesen OK, et al. Once-weekly semaglutide in adolescents with obesity. N Engl J Med. 2022;387:2245–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Ryan PM, Seltzer S, Hayward NE, Rodriguez DA, Sless RT, Hawkes CP. Safety and efficacy of glucagon-like peptide-1 receptor agonists in children and adolescents with obesity: A meta-analysis. J Pediatr. 2021;236:137–47.e13. [DOI] [PubMed] [Google Scholar]
- 98.Karagiannis T, Tsapas A, Athanasiadou E, Avgerinos I, Liakos A, Matthews DR, et al. GLP-1 receptor agonists and SGLT2 inhibitors for older people with type 2 diabetes: A systematic review and meta-analysis. Diabetes Res Clin Pract. 2021;174:108737. [DOI] [PubMed] [Google Scholar]
- 99.Traub J, Reiss L, Aliwa B, Stadlbauer V. Malnutrition in patients with liver cirrhosis. Nutrients. 2021;13:540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Montano-Loza AJ. Clinical relevance of sarcopenia in patients with cirrhosis. World J Gastroenterol. 2014;20:8061–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Sikirica M, Martin A, Wood R, Leith A, Piercy J, Higgins V. Reasons for discontinuation of GLP1 receptor agonists: Data from a real-world cross-sectional survey of physicians and their patients with type 2 diabetes. Diabetes Metab Syndr Obes. 2017;10:403–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Jensen SBK, Blond MB, Sandsdal RM, Olsen LM, Juhl CR, Lundgren JR, et al. Healthy weight loss maintenance with exercise, GLP-1 receptor agonist, or both combined followed by one year without treatment: A post-treatment analysis of a randomised placebo-controlled trial. eClinicalMedicine. 2024;69:102475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Wilding JPH, Batterham RL, Davies M, Van Gaal LF, Kandler K, Konakli K, et al. Weight regain and cardiometabolic effects after withdrawal of semaglutide: The STEP 1 trial extension. Diabetes Obes Metab. 2022;24:1553–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Verrastro O, Panunzi S, Castagneto-Gissey L, De Gaetano A, Lembo E, Capristo E, et al. Bariatric-metabolic surgery versus lifestyle intervention plus best medical care in non-alcoholic steatohepatitis (BRAVES): A multicentre, open-label, randomised trial. Lancet. 2023;401:1786–97. [DOI] [PubMed] [Google Scholar]
- 105.Lassailly G, Caiazzo R, Ntandja-Wandji LC, Gnemmi V, Baud G, Verkindt H, et al. Bariatric surgery provides long-term resolution of nonalcoholic steatohepatitis and regression of fibrosis. Gastroenterology. 2020;159:1290–301.e5. [DOI] [PubMed] [Google Scholar]
- 106.Loomba R, Ratziu V, Harrison SA, Loomba R, McFarlane SC, Tamaki N, et al. Expert panel review to compare FDA and EMA guidance on drug development and endpoints in nonalcoholic steatohepatitis. Gastroenterology. 2022;162:680–8. [DOI] [PMC free article] [PubMed] [Google Scholar]