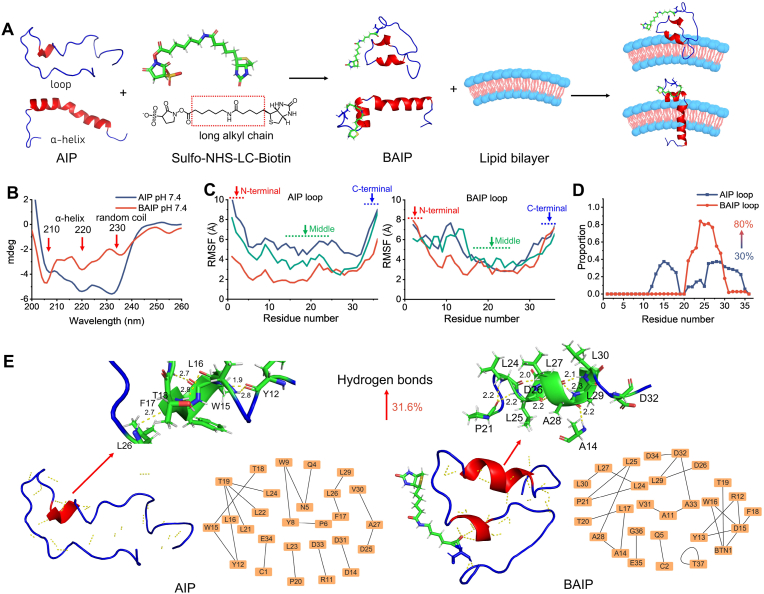
Fig. 2.
Design and engineering reconfiguration of WT pHLIP to BAIP. (A) Schematic of AIP modified with biotin and inserted into the phospholipid bilayer. (B) Circular dichroism spectra of AIP and BAIP at pH 7.4. (C) Root Mean Square Fluctuation (RMSF) of AIP and BAIP with loop conformation. The three spectra (red, blue, green) were the results of three independent simulation trajectories. RMSF refers to the structural change of an atom from its initial conformation over a period of time, reflecting the degree of freedom and flexibility of the atom. The results showed that the biotin modification had a notable effect not only on the N-terminal amino acid structure, but also on the middle and posterior amino acids of AIP when loop state was used as the initial model. (D) Proportion of each amino acid forming α-helix structure of AIP and BAIP with loop state. (E) Intramolecular hydrogen bonds of reconfigured AIP and BAIP with loop conformation analyzed by PyMOL, respectively. The enlarged hydrogen bonds diagrams were in AIP between residues L16-T18 and in BAIP between L25-L30, respectively. The unit of hydrogen bond length is Å.