Abstract
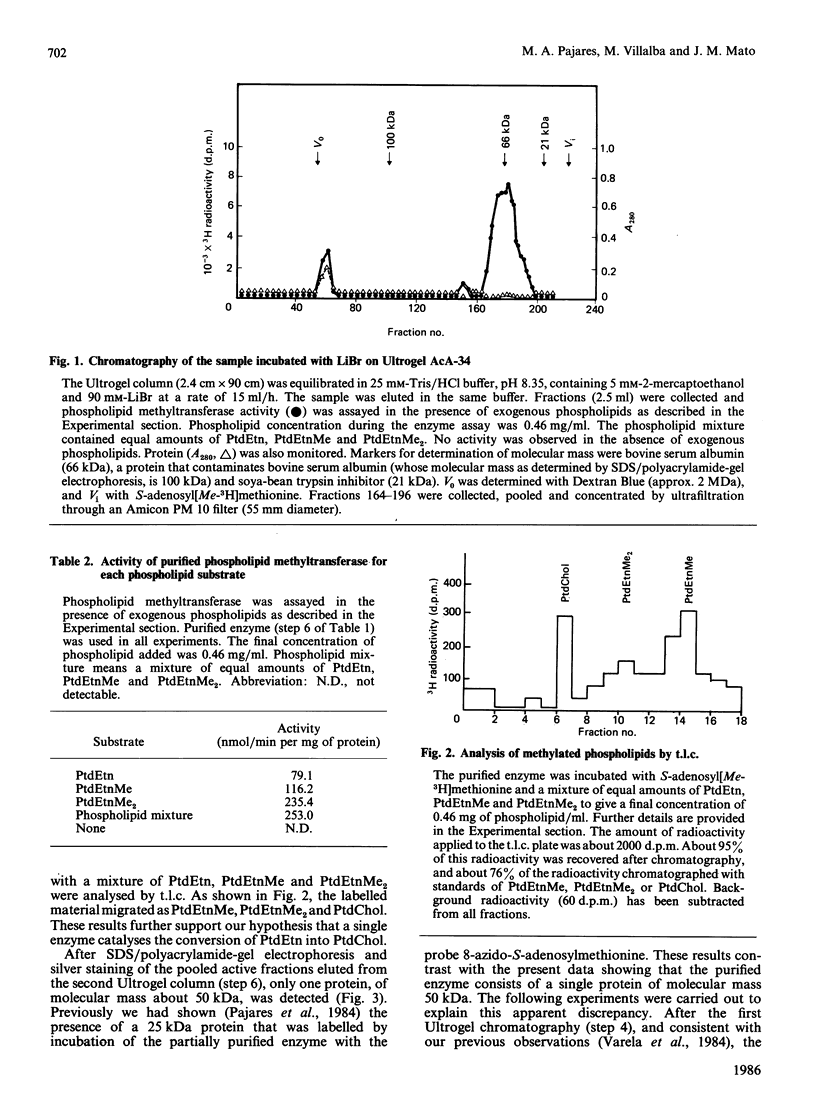
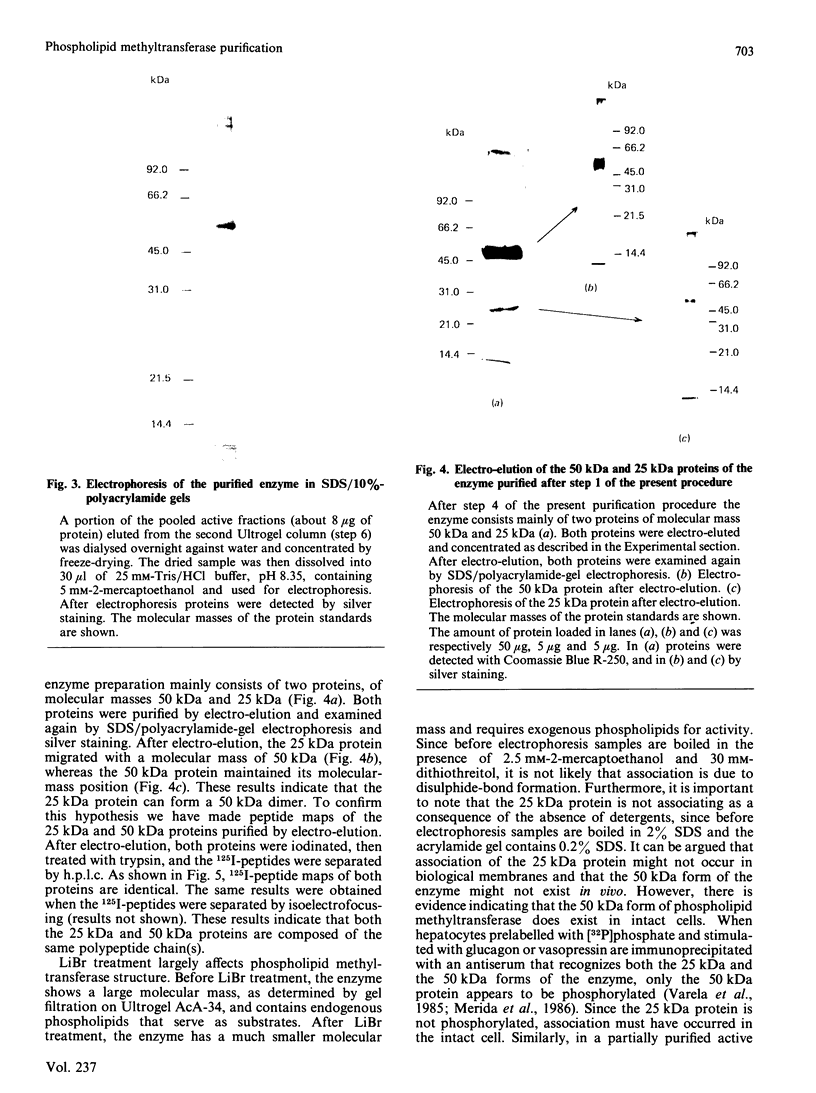
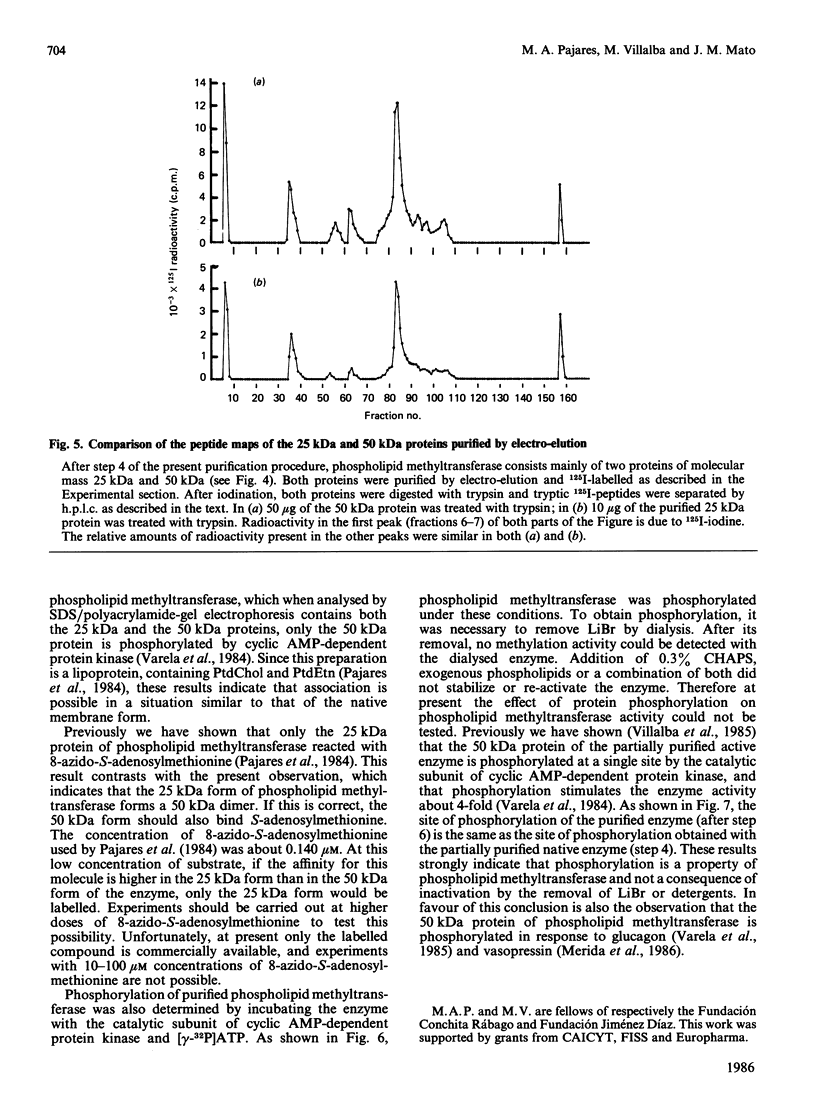
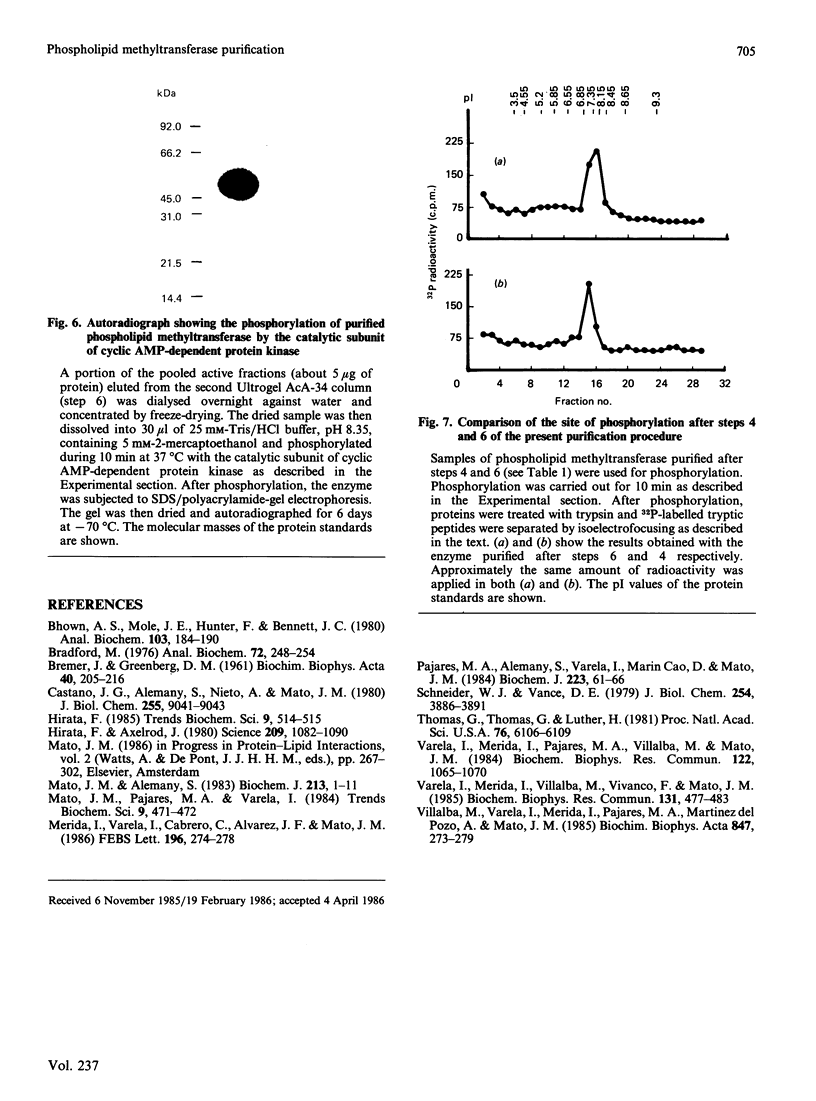
Phospholipid methyltransferase, the enzyme that converts phosphatidylethanolamine into phosphatidylcholine with S-adenosyl-L-methionine as the methyl donor, was purified to apparent homogeneity from rat liver microsomal fraction. When analysed by SDS/polyacrylamide-gel electrophoresis only one protein, with molecular mass about 50 kDa, is detected. This protein could be phosphorylated at a single site by incubation with [alpha-32P]ATP and the catalytic subunit of cyclic AMP-dependent protein kinase. A less-purified preparation of the enzyme is mainly composed of two proteins, with molecular masses about 50 kDa and 25 kDa, the 50 kDa form being phosphorylated at the same site as the homogeneous enzyme. After purification of both proteins by electro-elution, the 25 kDa protein forms a dimer and migrates on SDS/polyacrylamide-gel electrophoresis with molecular mass about 50 kDa. Peptide maps of purified 25 kDa and 50 kDa proteins are identical, indicating that both proteins are formed by the same polypeptide chain(s). It is concluded that rat liver phospholipid methyltransferase can exist in two forms, as a monomer of 25 kDa and as a dimer of 50 kDa. The dimer can be phosphorylated by cyclic AMP-dependent protein kinase.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bhown A. S., Mole J. E., Hunter F., Bennett J. C. High-sensitivity sequence determination of proteins quantitatively recovered from sodium dodecyl sulfate gels using an improved electrodialysis procedure. Anal Biochem. 1980 Mar 15;103(1):184–190. doi: 10.1016/0003-2697(80)90254-7. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Castaño J. G., Alemany S., Nieto A., Mato J. M. Activation of phospholipid methyltransferase by glucagon in rat hepatocytes. J Biol Chem. 1980 Oct 10;255(19):9041–9043. [PubMed] [Google Scholar]
- Hirata F., Axelrod J. Phospholipid methylation and biological signal transmission. Science. 1980 Sep 5;209(4461):1082–1090. doi: 10.1126/science.6157192. [DOI] [PubMed] [Google Scholar]
- Mato J. M., Alemany S. What is the function of phospholipid N-methylation? Biochem J. 1983 Jul 1;213(1):1–10. doi: 10.1042/bj2130001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mérida I., Varela I., Alvarez J. F., Cabrero C., Mato J. M. Vasopressin-stimulated phosphorylation of rat liver phospholipid methyltransferase in isolated hepatocytes. FEBS Lett. 1986 Feb 17;196(2):274–278. doi: 10.1016/0014-5793(86)80262-9. [DOI] [PubMed] [Google Scholar]
- Pajares M. A., Alemany S., Varela I., Marin Cao D., Mato J. M. Purification and photoaffinity labelling of lipid methyltransferase from rat liver. Biochem J. 1984 Oct 1;223(1):61–66. doi: 10.1042/bj2230061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider W. J., Vance D. E. Conversion of phosphatidylethanolamine to phosphatidylcholine in rat liver. Partial purification and characterization of the enzymatic activities. J Biol Chem. 1979 May 25;254(10):3886–3891. [PubMed] [Google Scholar]
- Varela I., Mérida I., Pajares M., Villalba M., Mato J. M. Activation of partially purified rat liver lipid methyltransferase by phosphorylation. Biochem Biophys Res Commun. 1984 Aug 16;122(3):1065–1070. doi: 10.1016/0006-291x(84)91199-9. [DOI] [PubMed] [Google Scholar]
- Varela I., Mérida I., Villalba M., Vivanco F., Mato J. M. Phospholipid methyltransferase phosphorylation by intact hepatocytes: effect of glucagon. Biochem Biophys Res Commun. 1985 Aug 30;131(1):477–483. doi: 10.1016/0006-291x(85)91827-3. [DOI] [PubMed] [Google Scholar]
- Villalba M., Varela I., Mérida I., Pajares M. A., Martínez del Pozo A., Mato J. M. Modulation by the ratio S-adenosylmethionine/S-adenosylhomocysteine of cyclic AMP-dependent phosphorylation of the 50 kDa protein of rat liver phospholipid methyltransferase. Biochim Biophys Acta. 1985 Dec 12;847(3):273–279. doi: 10.1016/0167-4889(85)90031-x. [DOI] [PubMed] [Google Scholar]





