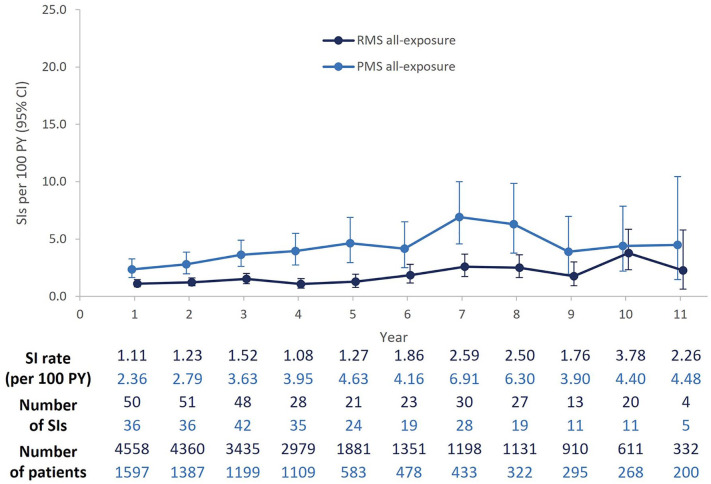
Figure 3.
Yearly rates of SIs in RMS and PMS populations, excluding COVID-19.
Pooled yearly SI rates from the 13 OCR clinical trials are presented. Patients with longer exposure (⩾6 years) are from the extension periods of phase II and phase III studies, including those originally randomized to the comparators interferon β-1a (OPERA) or placebo (ORATORIO) who switched to open-label OCR treatment. Patients with exposure >11 years (up to 14 years) are not represented in the graph; two SIs were reported in this group of patients beyond 11 years of treatment. CCOD, November 2022.
CCOD, clinical cut-off date; CI, confidence interval; COVID-19, coronavirus disease 2019; OCR, ocrelizumab; PMS, progressive multiple sclerosis; PY, patient years; RMS, relapsing multiple sclerosis; SI, serious infection.