Abstract
Background
Candidemia is an invasive mycosis with an increasing global incidence and high mortality rates in cancer patients. The production of biofilms by some strains of Candida constitutes a mechanism that limits the action of antifungal agents; however, there is limited and conflicting evidence about its role in the risk of death. This study aimed to determine whether biofilm formation is associated with mortality in cancer patients with candidemia.
Methods
This retrospective cohort study included patients treated at Peru’s oncologic reference center between June 2015 and October 2017. Data were collected by monitoring patients for 30 days from the diagnosis of candidemia until the date of death or hospital discharge. Statistical analyses evaluated the association between biofilm production determined by XTT reduction and mortality, adjusting for demographic, clinical, and microbiological factors assessed by the hospital routinary activities. Survival analysis and bivariate and multivariate Cox regression were used, estimating the hazard ratio (HR) as a measure of association with a significance level of p < 0.05.
Results
A total of 140 patients with candidemia were included in the study. The high mortality observed on the first day of post-diagnosis follow-up (81.0%) among 21 patients who were not treated with either antifungal or antimicrobial drugs led to stratification of the analyses according to whether they received treatment. In untreated patients, there was a mortality gradient in patients infected with non-biofilm-forming strains vs. low/medium and high-level biofilm-forming strains (25.0%, 66.7% and 82.3%, respectively, p = 0.049). In treated patients, a high level of biofilm formation was associated with increased mortality (HR, 3.92; 95% p = 0.022), and this association persisted after adjusting for age, comorbidities, and hospital emergency admission (HR, 6.59; CI: 1.87–23.24, p = 0.003).
Conclusions
The association between candidemia with in vitro biofilm formation and an increased risk of death consistently observed both in patients with and without treatment, provides another level of evidence for a possible causal association. The presence of comorbidities and the origin of the hospital emergency, which reflect the fragile clinical condition of the patients, and increasing age above 15 years were associated with a higher risk of death.
Keywords: Biofilms, Candidemia, Mortality, Cancer, Risk factors
Background
Candidemia is a nosocomial fungal infection worldwide [1–5] with rising numbers of cases due to the increase in immunocompromised patients in recent decades [6–10], and mortality rates between 30 and 50% among cancer patients [11–16]. There are known risk factors for death among patients with candidemia, such as advanced age, high Acute Physiology and Chronic Health Evaluation (APACHE II) score, presence of a central venous catheter (CVC), and lack of or inadequate antifungal treatment [17–26]. Additionally, the intrinsic characteristics of each Candida strain may also influence the clinical course of the disease [27–29].
Biofilms, which are microbial communities contained in an extracellular matrix, allow Candida yeasts to tolerate high concentrations of antifungal agents and evade the host immune response [30–35]. Evidence suggests that candidemia caused by biofilm-producing strains leads to an increased risk of death. Removal of CVCs, the main source of biofilm formation in clinical settings [36, 37], and treatment with echinocandins, an effective antifungal against Candida biofilms [38, 39], are associated with lower mortality in patients with candidemia [40–43]. However, these studies have not accounted for the role of biofilm formation by Candida spp. strains on disease outcomes.
In addition, there is conflicting evidence regarding biofilm formation and mortality in patients with candidemia [44–51], as some studies were unable to find an association [49–51], possibly due in part to sample size limitations and failure to adjust for confounding variables.
The high mortality of candidemia among immunocompromised patients, the potential additional mortality risk of biofilm-producing Candida strains, and the limited and contradictory evidence from previous studies, warrant a better understanding of the role of biofilms in the survival of patients with candidemia. Therefore, we evaluated whether the formation of biofilms is associated with increased mortality among cancer patients with candidemia.
Methods
Study design
In this retrospective cohort study, we compared the mortality of patients with candidemia caused by biofilm- and non-biofilm-forming strains. We analyzed samples and data collected from cancer patients at Peru’s National Oncology Reference Center (Instituto Nacional de Enfermedades Neoplasicas, INEN as per its acronym in Spanish) at candidemia diagnosis and during the following 30 days. The analyzed data was routinely collected in medical records by the treating physician, and in the INEN Microbiology Laboratory records. In addition, biofilm production assays were conducted on the strains collected during the study period. This test is not part of the laboratory routine evaluations.
Population and sample
The study population included hospitalized patients who were evaluated for blood stream infection and had at least one of the following: (1) fever or chills or leukopenia or leukocytosis, (2) Diagnosis of candidemia by positive blood cultures, and (3) Candida strain isolated at the INEN Microbiology Laboratory. Cases of repeated candidemia within a period of less than one month from a previous episode were excluded.
Microbiological methods
Peripheral blood samples were obtained from patients with suspected nosocomial infections by venipuncture or venipuncture and central venous catheter. Samples were taken into at least one vial of liquid culture medium and incubated in the BD BACTEC™ FX automated system (BD Diagnostics, Sparks, MD, USA) for up to 5 days [52–54]. Once a positive blood culture was detected by the system and yeasts were observed by Gram staining, they were isolated in the Sabouraud Glucose Agar culture medium. The identification of the isolate, at the genus and species levels, was carried out by morphological tests according to the Dalmaut Technique [55] on Rice Starch Agar and chromogenic tests using CHROMagar Candida (CHROMagar Microbiology, France) [56]. Confirmatory identification was performed by biochemical analysis of carbohydrates using the commercial API 20 C yeast identification method (bioMérieux) [57, 58]. In addition, fluconazole susceptibility was evaluated using agar-based methods: diffusion disk as screening method and E-test (AB Biodisk, Solna, Sweden) as a confirmed method, obtaining the categories of sensitive, sensitive dose-dependent, and resistant [59, 60]. Biofilm formation assays were performed using the standardized microplate method and quantified by reducing 2, 3-bis-(2-methoxy-4-nitro-5-sulfophenyl)-2 H-tetrazolium-5-carboxanilide (XTT) [61, 62]. Candida strains with optical densities (OD) < 0.1 were categorized as non-biofilm-formers and strains with OD ≥ 0.1 as biofilm-formers [45, 63, 64]. In addition, we categorized the levels of biofilm formation according to OD: no biofilms (< 0.1), low level (0.1 to 0.25), medium level (0.26 to 0.5), and high level (> 0.5). Low and medium level biofilm production categories were merged because the OD values of those two categories were similar and considerably lower than the OD values of the high category. Therefore, biofilm formation was compared between low/medium versus high levels.
Data collection and study variables
Laboratory information (Candida species and fluconazole sensitivity profile) was obtained from the INEN Laboratory Information System. The demographic information of the patients (sex and age), underlying medical conditions such as type of cancer, oncological diagnosis, comorbidities, hospital derivation, intensive care unit (ICU) stay, severe neutropenia status (absolute neutrophil count ≤ 0.5 × 109/L), invasive procedures (mechanical ventilation, parenteral nutrition), presence of central venous catheter (CVC), immunosuppressive therapy (corticosteroids), antimicrobial and antifungal therapy data were collected through review of medical records. Adequate antifungal therapy was defined as therapy received within 48 h of diagnosis and for at least five consecutive days [46, 65]. Biofilm production capacity of Candida isolates was evaluated at the Mycology Laboratory of the “Daniel Alcides Carrion” Instituto de Medicina Tropical, Universidad Nacional Mayor de San Marcos, as described in the text.
The outcome of interest, in-hospital mortality or survival, was measured from the time of candidemia diagnosis to the date of death (from any cause). Patients who survived after 30 days of follow-up or were discharged were censored and considered to be survivors in our analyses.
Statistical analysis
The relationship between in-hospital mortality and the differences across covariates was evaluated using the Chi2 test so mortality can be presented as a proportion using a direct, clear estimate. Survival curves were estimated with the Kaplan-Meier method and they were compared between covariate categories using the Log-rank test. Survival analysis was performed to identify possible differentiated patterns of mortality. Cox’s bivariate regression analysis was performed, estimating the crude mortality hazard ratio (HR), as a measure of association between in-hospital mortality, and the formation of biofilms as well as the HR for each of the covariates considered in the analysis. A manual forward stepwise process was used to build a parsimonious nested model, sequentially adding variables to the model based on likelihood ratio tests (p < 0.05). The goodness of fit of the model was determined through the analysis of Schoenfeld and Cox-Nell residuals and ties were handled using the partial likelihood method proposed by Efron. The assumption of proportionality of the hazards in the global model and for each covariate in the bivariate analyses were evaluated using graphical (Schoenfeld residuals and observed versus expected survival) and analytical (proportionality test) methods. The final multiple Cox regression model evaluated the possible association between the time to in-hospital mortality and biofilm formation, adjusted for all other covariables significantly associated with death. All data analysis was performed using Stata version 14.0 software (StataCorp, College Station, Texas 77845 USA).
Results
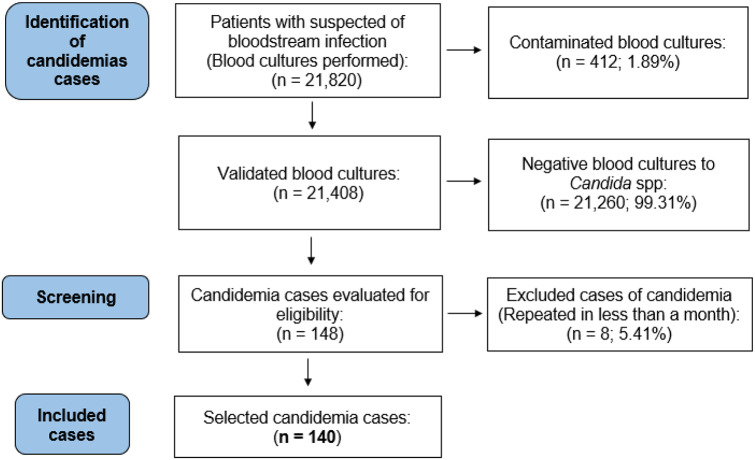
From June 2015 to October 2017, 21,820 blood cultures were evaluated at the INEN Microbiology Laboratory, resulting in 412 (1.89%) contaminated blood cultures. Among the valid cultures, 148 were positive for Candida spp. (0.69%), and were obtained from 137 patients. Three patients had two episodes of candidemia separated by more than one month and eight had positive blood cultures within less than one month of the previous episode. Therefore, the final available sample size was 140 patients with positive blood culture results for Candida spp. (Fig. 1).
Fig. 1.
Selection of the study sample
General characteristics of the sample
Most of the participants (58.6%) were adults between 16 and 59 years of age, and more than half were women (54.3%). Patients frequently presented with comorbidities (32.1%) and severe neutropenia (40.7%). The most common type of neoplasia was hematological malignancy (57.9%), and the most frequent oncological diagnoses were acute lymphocytic leukemia (25.7%) and gastrointestinal tumors (22.2%). A high percentage of the patients received antimicrobial therapy (79.3%) and immunosuppressive therapy (42.9%). In addition, 64.3% of the patients had a CVC and 22.1% had an ICU stay. Candidemia cases were treated with antifungals (76.4%), almost half of which were treated with antifungals with activity against biofilms, such as echinocandins and lipid formulation of amphotericin B (47.1%), and 62.1% of the patients received adequate antifungal therapy (therapy received within 48 h of diagnosis and for at least five consecutive days).
Subsequent identification of the isolates revealed that the predominant species were C. tropicalis (47.9%) and C. albicans complex (34.3%). More than a quarter of the isolates (27.2%) were resistant to fluconazole and the majority (75.7%) formed biofilms in vitro. Almost half of the patients (65, 46.4%) died within 30 days of the candidemia diagnosis. Most deaths occurred early, with almost a third of the fatalities taking place in the first day (32.3%). An additional 20% occurred between the second and fifth days, reaching 81.5% on day 15 of follow-up (Table 1).
Table 1.
Demographic, clinical, and microbiological characteristics and outcome of 140 cases of candidemia
| Characteristics | N (%) |
|---|---|
| Gender | |
| Male | 64 (45.7) |
| Female | 76 (54.3) |
| Age groups (years) | |
| 0–15 | 28 (20.0) |
| 16–59 | 82 (58.6) |
| ≥ 60 | 30 (21.4) |
| Type of cancer | |
| Solid tumors | 59 (42.1) |
| Hematological malignancies | 81 (57.9) |
| Oncological diagnosis | |
| Acute myeloid leukemia | 19 (13.6) |
| Acute lymphoid leukemia | 36 (25.7) |
| Non-Hodgkin lymphoma | 15 (10.7) |
| Gastrointestinal tumor | 31 (22.2) |
| Genitourinary tumor | 15 (10.7) |
| Others* | 24 (17.1) |
| Comorbidities | |
| No | 95 (67.9) |
| Infectious disease | 17 (12.2) |
| Respiratory disease | 10 (7.1) |
| Others** | 9 (6.4) |
| More than one comorbidity | 9 (6.4) |
| Hospital derivation | |
| Hospitalization | 132 (94.3) |
| Emergency | 8 (5.7) |
| Severe neutropenia | |
| No | 83 (59.3) |
| Yes | 57 (40.7) |
| Antimicrobial therapy | |
| No | 29 (20.7) |
| Yes | 111 (79.3) |
| Antifungal therapy | |
| No | 33 (23.6) |
| Yes | 107 (76.4) |
| Adequate antifungal therapy | |
| No | 53 (37.9) |
| Yes | 87 (62.1) |
| Anti-biofilm antifungal therapy | |
| No | 74 (52.9) |
| Yes | 66 (47.1) |
| ICU stay | |
| No | 109 (77.9) |
| Yes | 31 (22.1) |
| Presence of CVC | |
| No | 50 (35.7) |
| Yes | 90 (64.3) |
| Etiology | |
| C. albicans complex | 48 (34.3) |
| C. tropicalis | 67 (47.9) |
| C. parapsilosis complex | 9 (6.4) |
| C. glabrata complex | 10 (7.1) |
| Others*** | 6 (4.3) |
| Fluconazol resistance | |
| No | 91 (72.8) |
| Yes | 34 (27.2) |
| Biofilm formation level | |
| Non-formation | 34 (24.3) |
| Low-medium | 97 (69.3) |
| High | 9 (6.4) |
| Mortality rate | |
| No | 75 (53.6) |
| Yes | 65 (46.4) |
*(7) Head and neck cancer (6) chronic myeloid leukemia (3) Hodgkin lymphoma, osteosarcoma (2) Histiocytosis (1) liver cancer, mama cancer, skin cancer
**(3) Genitourinary disease, gastrointestinal disease (2) diabetes (1) Down syndrome
***(4) C. lusitaniae (2) Meyerozyma guilliermondii (C. guilliermondii) complex
Factors associated with in-hospital mortality
According to the medical records, mortality was at least 28% higher in patients > 15 years old (p = 0.034). Higher mortality was also observed in patients with comorbidities (64.4% vs. 37.9%, p = 0.003), in those admitted for emergencies (87.5% vs. 43.9%, p = 0.016), and in patients who did not receive therapy and/or received inappropriate therapy (60.4% vs. 37.9%, p = 0.010). In addition, patients with candidemia that formed biofilms had a marginally higher mortality (50.9% vs. 32.3%, p = 0.059), with a significant positive correlation between higher biofilm formation and higher mortality (p = 0.033). On the other hand, we observed lower mortality in both patients that received antifungal therapy (41.1% vs. 63.6%, p = 0.023) and those who received antimicrobial therapy (41.4% vs. 65.5%, p = 0.021). Interestingly, mortality rates were very similar in treated and non-treated patients for both types of therapy, since according to the clinical records, both antifungal and antimicrobial therapy prescription in the same patient coincided much more often that they differed (90% vs. 10%, p < 0.001). Patients with CVC also had lower mortality (40.0% vs. 58.0%, p = 0.041), as well as those receiving adequate antifungal therapy (37.9% vs. 60.4%, p = 0.010). In addition, there was no proportionality of hazards for antimicrobial (p = 0.001) or antifungal (p = 0.033) treatment, while the hazards were proportional for all other covariates. (Table 2). In an additional analysis, we found that biofilm production differed by Candida species (p = 0.015), with more frequent biofilm-producing strains in C. tropicalis (57/67 = 85.1%) and fewer among C. parapsilosis complex (4/9 = 44.4%). Among the nine high-level biofilm-producing strains, seven were identified as C. tropicalis and two as C. glabrata complex.
Table 2.
Demographic, clinical, and microbiological characteristics of 140 cases of candidemia according to mortality, and proportional hazards assessment
| Variables | Survivors N (%) | Deceased N (%) | p value | Proportionality (p value) |
|---|---|---|---|---|
| Gender | 0.560 | 0.163 | ||
| Male | 36 (56.3) | 28 (43.7) | ||
| Female | 39 (51.3) | 37 (48.7) | ||
| Age groups (years) | 0.034 | 0.624 | ||
| 0–15 | 23 (82.1) | 5 (17.9) | ||
| 16–59 | 36 (43.9) | 46 (56.1) | ||
| ≥ 60 | 36 (43.9) | 46 (56.1) | ||
| Type of cancer | 0.132 | 0.333 | ||
| Solid tumors | 36 (61.0) | 23 (39.0) | ||
| Hematological malignancies | 39 (48.1) | 42 (51.9) | ||
| Oncological diagnosis | 0.674 | 0.393 | ||
| Acute myeloid leukemia | 11 (57.9) | 8 (42.1) | ||
| Acute lymphoid leukemia | 19 (52.8) | 17 (47.2) | ||
| Non-Hodgkin lymphoma | 6 (40.0) | 9 (60.0) | ||
| Gastrointestinal tumor | 20 (64.5) | 11 (35.5) | ||
| Genitourinary tumor | 7 (46.7) | 8 (53.3) | ||
| Others* | 12 (50.0) | 12 (50.0) | ||
| Comorbidity | 0.003 | 0.745 | ||
| No | 59 (62.1) | 36 (37.9) | ||
| Yes | 16 (35.6) | 29 (64.4) | ||
| Type of comorbidity | 0.011 | 0.426 | ||
| No | 59 (62.1) | 36 (37.9) | ||
| Infectious disease | 6 (35.3) | 11 (64.7) | ||
| Respiratory disease | 1 (10.0) | 9 (90.0) | ||
| Others** | 5 (55.6) | 4 (44.4) | ||
| More than one comorbidity | 4 (44.4) | 5 (55.6) | ||
| Hospital derivation | 0.016 | 0.993 | ||
| Hospitalization | 74 (56.1) | 58 (43.9) | ||
| Emergency | 1 (12.5) | 7 (87.5) | ||
| Severe neutropenia | 0.223 | 0.256 | ||
| No | 48 (57.8) | 35 (42.2) | ||
| Yes | 27 (47.4) | 30 (52.6) | ||
| Antimicrobial therapy | 0.021 | 0.001 | ||
| No | 10 (34.5) | 19 (65.5) | ||
| Yes | 65 (58.6) | 46 (41.4) | ||
| Antifungal therapy | 0.023 | 0.033 | ||
| No | 12 (36.4) | 21 (63.6) | ||
| Yes | 63 (58.9) | 44 (41.1) | ||
| Adequate antifungal therapy | 0.010 | 0.034 | ||
| No | 21 (39.6) | 32 (60.4) | ||
| Yes | 54 (62.1) | 33 (37.9) | ||
| Anti-biofilm antifungal therapy | 0.370 | 0.041 | ||
| No | 37 (50.0) | 37 (50.0) | ||
| Yes | 38 (57.6) | 28 (42.4) | ||
| ICU stay | 0.141 | 0.503 | ||
| No | 62 (56.9) | 47 (43.1) | ||
| Yes | 13 (41.9) | 18 (58.1) | ||
| Presence of CVC | 0.041 | 0.087 | ||
| No | 21 (42.0) | 29 (58.0) | ||
| Yes | 54 (60.0) | 36 (40.0) | ||
| Etiology | 0.541 | 0.549 | ||
| C. albicans complex | 24 (50.0) | 24 (50.0) | ||
| Candida no albicans complex | 51 (55.4) | 41 (44.6) | ||
| Candida species | 0.332 | 0.264 | ||
| C. albicans complex | 24 (50.0) | 24 (50.0) | ||
| C. tropicalis | 33 (49.3) | 34 (50.7) | ||
| C. parapsilosis complex | 6 (66.7) | 3 (33.3) | ||
| C. glabrata complex | 7 (70.0) | 3 (30.0) | ||
| Others | 5 (83.3) | 1 (16.7) | ||
| Fluconazol resistance | 0.834 | 0.731 | ||
| No | 50 (55.0) | 41 (45.0) | ||
| Yes | 18 (52.9) | 16 (47.1) | ||
| Biofilm formation | 0.059 | 0.790 | ||
| No | 23 (67.7) | 11 (32.3) | ||
| Yes | 52 (49.1) | 54 (50.9) | ||
| Biofilm formation level | 0.033 | 0.652 | ||
| Non-formation | 23 (67.7) | 11 (32.3) | ||
| Low-medium | 49 (50.5) | 48 (49.5) | ||
| High | 3 (33.3) | 6 (66.7) |
Survival analysis
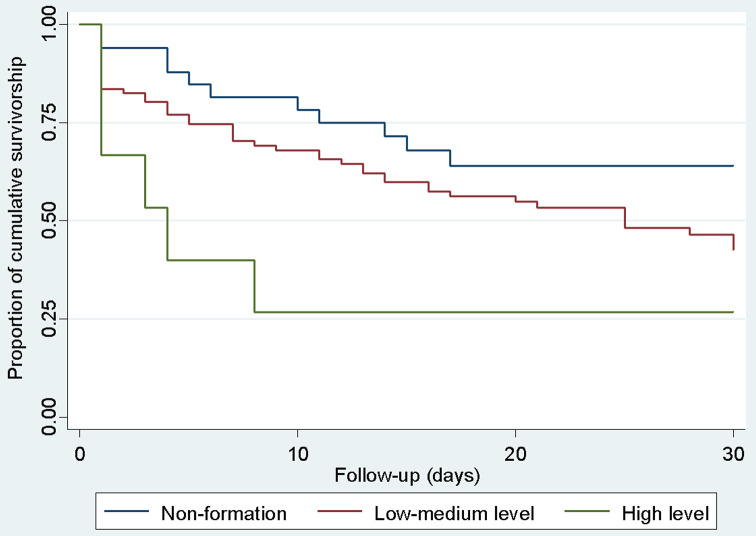
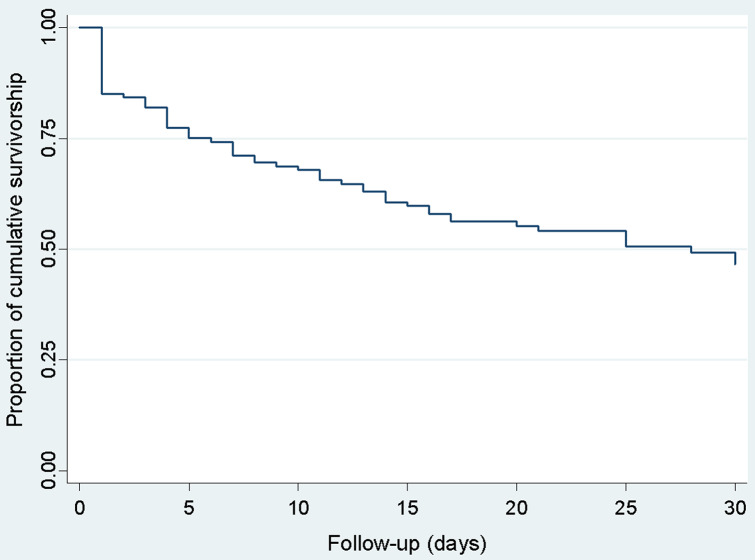
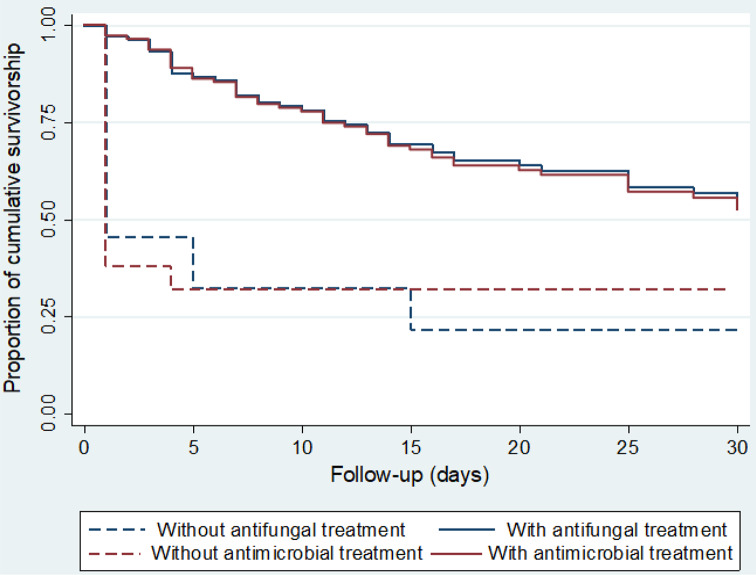
Decreased 30-day survival was observed with higher biofilm production of Candida isolates. (Fig. 2). A substantial decrease in survival was observed on the first day of follow-up, while deaths were more sporadic after day two, and survival decreased more slowly and progressively since (Fig. 3). This differentiated pattern of mortality early during follow-up and the lack of proportional hazards for antifungal and antimicrobial treatment, warranted for stratification by treatment status and estimation of separate survival curves for patients with different antifungal and antimicrobial treatment. For both types of therapy, the survival curves of the patients showed substantially higher mortality on the first day of follow-up among untreated patients but a lower, more homogeneous mortality rate across the 30-day period in treated patients (Fig. 4). This survival pattern was compatible with the disproportionate hazards associated with antifungal and antimicrobial treatment. When exploring in detail the characteristics of patients who died on the first day, the vast majority of deaths occurred in patients who did not receive either antifungal or antimicrobial therapy (17/21 = 81.0%, Table 3). In contrast, deaths after the second day occurred almost exclusively in patients who received both therapies, and became more progressive.
Fig. 2.
Survival of patients with candidemia according to the level of biofilm formation of the isolates estimated by the Kaplan Meier method
Fig. 3.
Overall survival of patients with candidemia estimated by the Kaplan Meier method
Fig. 4.
Survival of patients with candidemia according to having received antifungal and antimicrobial treatment estimated by the Kaplan Meier method
Table 3.
Day of death and total number of deaths during follow-up, stratified according to having received antifungal and antimicrobial treatment
| Groups | No treatment (n = 24) |
Only antimicrobial treatment (n = 9) |
Only antifungal treatment (n = 5) |
Both treatments (n = 102) |
Total (n = 140) |
|---|---|---|---|---|---|
| Total deaths | 17 (71%) | 4 (44%) | 2 (40%) | 42 (41%) | 65 (46%) |
| Death day | |||||
| 1 | 17 (100%) | 1 (25%) | 1 (50%) | 2 (5%) | 21 (32%) |
| 2–5 | 0 (0%) | 2 (50%) | 1 (50%) | 10 (24%) | 13 (20%) |
| 6–15 | 0 (0%) | 1 (25%) | 0 (0%) | 18 (43%) | 19 (29%) |
| 16–30 | 0 (0%) | 0 (0%) | 0 (0%) | 12 (28%) | 12 (19%) |
| Total no deaths | 7 | 5 | 3 | 60 | 75 |
| % Deaths at the day 1 | 17/24 (71%) | 1/9 (11%) | 1/5 (20%) | 2/102 (2%) | 21/140 (15%) |
Given the large difference in first-day mortality between untreated and treated patients, we hypothesized that treatment could be an effect modifier for the association between biofilm formation and survival. Therefore, we stratified by treatment, following Kleinbaum recommendations [66], to be able to apply Cox regression in treatment-defined groups with proportional hazards. Therefore, the results were analyzed separately in two strata:1) patients that did not receive antifungal or antimicrobial treatment, and 2) patients who received antifungal and/or antimicrobial treatment. The association with mortality was analyzed dichotomously in the first stratum because deaths were mostly concentrated on the first day, while in the second stratum the assumptions for Cox regression as a time-to-event approach were valid since mortality occurred more progressively.
Mortality in patients with candidemia according to treatment stratum
Stratum 1: patients without antimicrobial or antifungal treatment (n = 24)
Out of the 24 patients who did not receive these two treatments, 17 (70.8%) died. Higher mortality was observed in patients with hematological malignancies (100.0% vs. 46.1%, p = 0.006) and among patients infected with low-medium and high-level biofilm-forming strains of Candida spp. versus infections with strains that did not form biofilms (82.3% vs. 25.0% and 66.7% vs. 25.0%, p = 0.049, Table 4). No other statistically significant differences in mortality were observed, perhaps because of the small sample size and the resulting low power that also prevented exploration of possible multivariate associations.
Table 4.
Factors associated with mortality in patients without antimicrobial or antifungal treatment (n = 24)
| Variables | Survivors N (%) | Deceased N (%) | p value |
|---|---|---|---|
| Gender | 1.000 | ||
| Male | 4 (33.3) | 8 (66.7) | |
| Female | 3 (25.0) | 9 (75.0) | |
| Age groups (years) | 0.077 | ||
| 0–15 | 0 (0) | 0 (0) | |
| 16–59 | 3 (17.7) | 14 (82.4) | |
| ≥ 60 | 4 (57.1) | 3 (42.9) | |
| Type of cancer | 0.006 | ||
| Solid tumors | 7 (53.9) | 6 (46.1) | |
| Hematological malignancies | 0 (0) | 11 (100.0) | |
| Comorbidity | 0.065 | ||
| No | 7 (41.2) | 10 (58.8) | |
| Yes | 0 (0) | 7 (100.0) | |
| Hospital derivation | 1.000 | ||
| Hospitalization | 7 (31.8) | 15 (68.2) | |
| Emergency | 0 (0) | 2 (100.0) | |
| Severe neutropenia | 0.130 | ||
| No | 7 (38.9) | 11 (61.1) | |
| Yes | 0 (0) | 6 (100.0) | |
| ICU stay | 1.000 | ||
| No | 7 (30.4) | 16 (69.6) | |
| Yes | 0 (0) | 1 (100.0) | |
| Presence of CVC | 0.507 | ||
| No | 6 (27.3) | 16 (72.7) | |
| Yes | 1 (50.0) | 1 (50.0) | |
| Etiology | 1.000 | ||
| C. albicans complex | 3 (33.3) | 6 (66.7) | |
| Candida no albicans complex | 4 (26.7) | 11 (73.3) | |
| Fluconazol resistance | 0.826 | ||
| No | 3 (23.1) | 10 (76.9) | |
| Yes | 3 (42.9) | 4 (57.1) | |
| Biofilm formation | 0.059 | ||
| No | 3 (75.0) | 1 (25.0) | |
| Yes | 4 (20.0) | 16 (80.0) | |
| Biofilm formation level | 0.049 | ||
| Non-formation | 3 (75.0) | 1 (25.0) | |
| Low-medium | 3 (17.7) | 14 (82.3) | |
| High | 1 (33.3) | 2 (66.7) |
Stratum 2: patients who received antimicrobial and/or antifungal treatment (n = 116)
A total of 48 patients died within this stratum (41.4%). Bivariate analysis showed that there was a higher instantaneous risk of death in patients in 16–59 and 60 + years old compared to 0–15 years old (HR 3.61, p = 0.008; HR 3.87, p = 0.012, respectively), with some comorbidity (HR 1.82; p = 0.038), hospital emergency (HR 4.84; p = 0.001), receiving inadequate antifungal therapy (HR 2.18; p = 0.013), mechanical ventilation (HR 1.83; p = 0.037), and infection with high-level biofilm-forming strains of Candida (HR 3.92; p = 0.022).
Exploratory multiple regression analyses in this second stratum found independent associations between a greater instantaneous risk of death and older age (16–59 years with HR 4.27; p = 0.003 and 60 + years with HR 5.88; p = 0.001 compared to 0–15 years), presence of comorbidities (HR 2.72; p = 0.001), hospital emergency derivation (HR 7.58; p < 0.001), and a high level of biofilm formation (HR 6.59; p = 0.003). Hazards were proportional for each covariate evaluated and for all covariates together (p > 0.2 and p = 0.848, respectively, Table 5).
Table 5.
Factors associated with mortality in patients with antimicrobial and antifungal treatment (n = 116)
| Variables | Raw model | Adjusted model† | |||||
|---|---|---|---|---|---|---|---|
| HR | CI 95% | p value | Propor. | HR | CI 95% | p value | |
| Gender | 0.148 | ||||||
| Male | Ref. | Ref. | |||||
| Female | 1.17 | 0.66–2.10 | 0.576 | 0.94 | 0.50–1.76 | 0.846 | |
| Age groups (years) | |||||||
| 0–15 | Ref. | 0.977 | Ref. | ||||
| 16–59 | 3.61 | 1.40–9.27 | 0.008 | 4.27 | 1.64–11.11 | 0.003 | |
| ≥ 60 | 3.87 | 1.34–11.16 | 0.012 | 5.88 | 1.98–17.44 | 0.001 | |
| Type of cancer | 0.161 | ||||||
| Solid tumors | Ref. | Ref. | |||||
| Hematological malignancies | 1.02 | 0.56–1.83 | 0.958 | 1.31 | 0.66–2.57 | 0.437 | |
| Comorbidity | 0.540 | ||||||
| No | Ref. | Ref. | |||||
| Yes | 1.82 | 1.03–3.22 | 0.035 | 2.72 | 1.47–5.01 | 0.001 | |
| Hospital derivation | 0.741 | ||||||
| Hospitalization | Ref. | Ref. | |||||
| Emergency | 4.84 | 1.88–12.45 | 0.001 | 7.58 | 2.67–21.49 | < 0.001 | |
| Severe neutropenia | 0.374 | ||||||
| No | Ref. | Ref. | |||||
| Yes | 1.19 | 0.67–2.09 | 0.553 | 1.29 | 0.72–2.34 | 0.394 | |
| Antimicrobial therapy | 0.189 | ||||||
| No | Ref. | Ref. | |||||
| Yes | 0.76 | 0.18–3.13 | 0.702 | 0.41 | 0.95–1.79 | 0.236 | |
| Antifungal therapy | 0.958 | ||||||
| No | Ref. | Ref. | |||||
| Yes | 0.43 | 0.15–1.20 | 0.107 | 0.38 | 0.12–1.15 | 0.086 | |
| Adequate antifungal therapy | 0.295 | ||||||
| No | Ref. | Ref. | |||||
| Yes | 0.46 | 0.25–0.85 | 0.013 | 0.61 | 0.30–1.20 | 0.151 | |
| Anti-biofilm antifungal therapy | 0.405 | ||||||
| No | Ref. | Ref. | |||||
| Yes | 0.88 | 0.50–1.57 | 0.670 | 0.93 | 0.52–1.68 | 0.816 | |
| ICU stay | 0.614 | ||||||
| No | Ref. | Ref. | |||||
| Yes | 1.60 | 0.88–2.89 | 0.121 | 1.51 | 0.80–2.85 | 0.208 | |
| Presence of CVC | 0.754 | ||||||
| No | Ref. | Ref. | |||||
| Yes | 0.67 | 0.35–1.26 | 0.214 | 0.90 | 0.45–1.80 | 0.763 | |
| Etiology | 0.568 | ||||||
| C. albicans complex | Ref. | Ref. | |||||
| Candida no albicans complex | 0.77 | 0.42–1.38 | 0.372 | 0.75 | 0.41–1.39 | 0.361 | |
| Fluconazol resistance | 0.477 | ||||||
| No | Ref. | Ref. | |||||
| Yes | 1.05 | 0.62–1.76 | 0.861 | 1.59 | 0.89–2.84 | 0.116 | |
| Biofilm formation | 0.340 | ||||||
| No | Ref. | Ref. | |||||
| Yes | 1.39 | 0.69–2.78 | 0.351 | No analizable | |||
| Biofilm formation level | 0.208 | ||||||
| Non-formation | Ref. | Ref. | |||||
| Low-medium | 1.30 | 0.64–2.62 | 0.472 | 1.30 | 0.62–2.75 | 0.492 | |
| High | 3.92 | 1.22–12.57 | 0.022 | 6.59 | 1.87–23.24 | 0.003 | |
Discussion
We observed a significant association between high levels of in vitro biofilm formation and increased mortality among candidemia cases. This association was consistently present both in patients who did not receive antimicrobial nor antifungal treatment in whom mortality was high and deaths occurred very early, and also in patients who received at least one of these treatments, among whom mortality was lower and occurred progressively during follow-up. In the latter group, the association remained significant after multiple regression adjustment.
Our findings confirm hypotheses raised by previous studies that observed higher mortality in candidemia with biofilm formation [44–48], but contradict other studies that found no significant association [49–51]. In both cases, the results may be inconclusive because the studies had small sample sizes [44, 47, 49, 50] and did not include potential confounding variables in their analysis, such as patients’ baseline clinical status [44, 48–50], presence of comorbidities [46, 47], administration of antifungal therapy, and in vitro resistance of isolates to fluconazole [49, 50]. Finally, no previous studies [44–51] had assessed the timing and temporal pattern of survival in these frail patients, which was critical to detect basal differences in the survival probability.
Our study design and analysis attempted to overcome the challenges faced by previous studies, and our findings suggest a possible role of biofilm formation on increased mortality among patients with candidemia. There are some possible mechanisms that could explain how candidemia with high biofilm formation could lead to higher mortality. For example, antifungal drugs penetrate poorly into biofilm structures, allowing Candida spp. yeasts to resist their action [30]. Additionally, biofilms can act as a physical barrier that prevents phagocytosis, allow yeasts to evade the host immune response, and alters the profile of cytokines secreted by immune cells [35]. Therefore, infections with candida strains that form biofilms could hinder effective treatment and represent a risk factor for a complicated clinical course and potentially fatal outcomes. Although candidemia mortality rate is lower outside cancer patients, biofilm formation remains a concern as it can play a role in fatal outcomes in non-tumor populations. Three studies documented lower but non-negligible mortality rates among non-tumor hospitalized patients with candidemia compared to patients with malignancies. Among 126 Korean ICU patients, mortality was lower among non-tumor patients (43/81; 53.1% versus 32/45; 71.1%, p = 0.048) and remained lower after adjusting for hemodialysis, mycological failure, and septic shock (OR, 0.12, 95% CI: 0.03–0.45, p = 0.002) [67]. Similarly, in 60 hospitalized patients from a 750-bed Korean tertiary medical center, mortality was lower in non-cancer patients (6/23; 26.1% versus 19/37; 51.4%, p = 0.05), and remained lower after adjusting for APACHE II score and receipt of antifungal treatment (OR, 0.07; 95% CI, 0.01–0.40; p = 0.003) [68]. Finally, among 341 patients with candidemia at 14 major hospitals in Spain, mortality was lower in patients without hematologic malignancy (27; 13% versus 9; 24%, RR 0.5, p = 0.1), and remained lower after excluding C. parapsilosis cases and deaths occurring at days 1 to 2, and adjusting for severity of illness category (OR, 0.29; 95% CI, 0.01 to 0.91, p = 0.03) [69]. In summary, there is considerable mortality in hospitalized patients with candidemia, even in the absence of malignancies, an important risk factor. Prospective studies of potential mechanisms of increased virulence, such as drug inactivation and immune evasion, could help to elucidate the role of biofilms in the clinical course of candidemia.
Our survival analysis identified a subgroup of patients with very high, early mortality, suggesting they had a highly vulnerable baseline clinical condition at cohort entry and a worse prognosis [70, 71]. The identification of two groups with dramatic differences in mortality rates and time of death suggests a differential risk of death associated with antifungal or antimicrobial treatment. Therefore, the study methodology was adjusted by introducing stratification by treatment, but higher mortality associated with biofilm formation was consistently observed, regardless of the treatment received and the level of associated mortality. The high mortality observed in patients infected with biofilm-producing strains but were not treated is important evidence that suggests how severe this condition can be among frail, vulnerable patients. This is an important finding that should be confirmed in future studies with larger sample sizes.
The lower mortality rate observed among patients receiving antimicrobial therapy has not been described previously and may be an artificial effect of prophylactic treatment in the hospital. Patients with candidemia receiving antifungal drugs often receive antimicrobial therapy as well because they are at risk of developing severe bacterial infections [72]. In our study, almost all patients who received an antifungal drug also received antimicrobial therapy (102/107 = 95.3%), and most patients who did not receive an antifungal drug also did not receive antimicrobial therapy (24/33 = 72.7%). However, it is unlikely that the antimicrobial treatment received could directly reduce mortality from candidemia.
We observed that late antifungal treatment or inadequate dosing was associated with higher mortality in candidemia, in consistency with studies showing the efficacy of antifungal therapy in candidemia depends on whether or not it is properly administered [26, 40, 43]. Antifungal therapy can reduce mortality, particularly if administered within 48 h of diagnosis and for at least 5 consecutive days. Similarly, the lipid formulation of amphotericin B and echinocandins has documented activity against Candida spp. biofilms [38, 39]. However, we found no association between the use of these antifungal agents and mortality from candidemia. Limited statistical power may have prevented detecting small reductions in mortality associated with antifungal therapies against biofilms. Evaluation of these antifungal agents in prospective studies with a larger sample size could better document the effect of these antifungal agents on candidemia caused by biofilm-producing strains. Antifungal prophylaxis was not part of oncologic therapeutic management at the Institute at that time except for fluconazole and posaconazole prophylaxis for patients with acute myeloid leukemia, which accounted for only 13.6% of the study sample.
On the other hand, confirmatory laboratory diagnosis of candidemia is mainly limited by performing microbiological culture of multiple blood samples. The Candida spp isolates in our study are very likely to be the true cause of the candidemia since more than 90% of the blood cultures were performed with multiple specimens from at least two sites sampling from both arms and had concordant results, reducing the possibility of skin-contaminated samples. Also, a second infection was detected in only 12.2% of the patients. On the other hand, non-culture-based tests would have been useful in diagnosing candidemia, such as the (1–3)-β-D-glucan test, but limited resources prevented their use. However, having multiple, concordant results from different cultures, there are lower chances of contamination and less need for further supplementary testing.
Candidemia is the most common invasive mycosis and has challenging diagnosis. Its clinical course of this fungal infection is severe and life-threatening, with a high mortality rate among immunocompromised populations. Therefore, early suspicion and consideration among the differential diagnoses of hospital-acquired infections are critical in at-risk patients, such as our oncologic population. The role of empirical treatment and antifungal prophylaxis in resource-limited settings requires further research. Adulthood, age older than 15 years [18, 24], and the presence of some comorbidity [73–75] were identified, similar to previous studies, along with emergency hospital admission as factors strongly associated with higher mortality from candidemia. These factors likely characterize frail patients with candidemia with a worse prognosis. Such empirical results reflect the important role of patients’ baseline clinical condition in their potential survival and should be further investigated in future prospective studies.
The study had some particularities that must be considered when interpreting the results, but do not invalidate our conclusions. First, the stage and status of the cancer were not evaluated, variables that may provide an indication of the severity of the patient’s clinical condition. However, other variables capture the potential frailty of the baseline clinical condition, such as treatment in the hospital emergency department, presence of chronic disease, and advanced age. Second, patient health status severity was not assessed because this is not routinely done for all patients treated at INEN. In addition, there is no uniform scale for assessing severity in patients admitted to different hospital units. For example, the scale APACHE II, cannot be used in patients outside the ICU (77.9% of the sample) or in the pediatric population (20.0% of the sample). In addition, the effective sample size was partially reduced by separately analyzing patients who received antifungal or antimicrobial treatment (82.9% of the sample), then no conclusions could be drawn about the analysis of patient mortality by antifungal and antimicrobial treatment received due to the small sample size. Although several significant differences were found, including those related to the study hypothesis, there might be other associations that statistical power was insufficient to detect. Finally, we have no information on CVC removal, which some studies have found to be associated with survival among patients with candidemia, although it is difficult to understand retrospectively the reasons for CVC removal among hospitalized patients. CVC removal in patients with good prognosis may aim to prevent hospital-acquired infections but in patients under palliative therapy may be the beginning of less invasive interventions.
Conclusions
The association between candidemia with in vitro biofilm formation and an increased risk of death consistently observed in both treated and untreated patients, provides an additional level of evidence for a possible causal association.
Survival analysis in a time-to-event study is essential to detect differences in the probabilities of developing the outcome. In our study, this analysis allowed us to ensure an adequate assessment of the true risk of death that patients had since their entry into the cohort.
The presence of comorbidities and the origin of the hospital emergency, which reflect the fragile clinical condition of the patients, and increasing age above 15 years were associated with a higher risk of death.
Acknowledgements
The authors thank professors of the master’s program for their contributions in the study design, data analysis, and manuscript preparation. AGL and the Master’s program are supported by program D43 TW007393 “Emerge, Emerging Diseases Epidemiology Research Training,” supported by the Fogarty International Center of the United States National Institutes of Health. We thank the Belgian Development Agency - CTB grant program of Belgium Government for funding the master’s degree program, Dr. Gustavo Giusiano, from the Instituto de Medicina Regional at Universidad Nacional del Nordeste, and Dr. Alexis Holguín, from the Infectious Disease Department at INEN, for advice on laboratory and hospital data analysis, respectively.
Abbreviations
- APACHE
Acute Physiology and Chronic Health Evaluation
- CVC
Central venous catheter
- INEN
Instituto Nacional de Enfermedades Neoplasicas
- OD
Optical densities
- ICU
Intensive care unit
- HR
Hazard ratio
Author contributions
This research was the thesis of FV-C for his Masters of Science in Epidemiological Research at Universidad Peruana Cayetano Heredia. FV and AGL conception and design of the study; FV, JG, VB, IC, CM and AM biofilm assay; FV, IC, CM and AM data collection; FV and AGL data analysis; FV, AGL, JG and VB writing, review and editing of the manuscript. All authors read and approved the final version of the manuscript.
Funding
All activities involving this study were self-financing without any organizational support.
Data availability
Data is provided within the manuscript or supplementary information files.
Declarations
Ethical approval
This is a secondary analysis of clinical and microbiological data available in the hospital records and primary analysis derived from the biofilm assay in Candida isolates, respectively; performed without any use of human specimens nor contact with human subjects. The data was compiled and analyzed anonymously using unique study codes at all times, and the investigators did not have access to any personal identifiers. Prior to its execution, the study protocol was approved by the INEN Research Department Protocol Review Committee (INEN 16–45) and by the Universidad Peruana Cayetano Heredia Ethics Committee (SIDISI 101763). The research was conducted respecting all ethical principles outlined in the Helsinki Declaration.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Pfaller MA, Diekema D. Epidemiology of invasive candidiasis: a persistent public health problem. Clin Microbiol Rev. 2007;20(1):133–63. 10.1128/CMR.00029-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pappas PG. Invasive candidiasis. Infect Disease Clin. 2006;20(3):485–506. 10.1016/j.idc.2006.07.004. [DOI] [PubMed] [Google Scholar]
- 3.Sievert DM, Ricks P, Edwards JR, Schneider A, Patel J, Srinivasan A, Fridkin S. Antimicrobial-resistant pathogens associated with healthcare-associated infections summary of data reported to the National Healthcare Safety Network at the Centers for Disease Control and Prevention, 2009–2010. Infect Control Hosp Epidemiol. 2013;34(1):1–14. 10.1086/668770. [DOI] [PubMed] [Google Scholar]
- 4.Wisplinghoff H, Bischoff T, Tallent SM, Seifert H, Wenzel RP, Edmond MB. Nosocomial bloodstream infections in US hospitals: analysis of 24,179 cases from a prospective nationwide surveillance study. Clin Infect Dis. 2004;39(3):309–17. 10.1086/421946. [DOI] [PubMed] [Google Scholar]
- 5.Nucci M, Queiroz-Telles F, Alvarado-Matute T, Tiraboschi IN, Cortes J, Zurita J, Echevarria. J. I. Epidemiology of candidemia in Latin America: a laboratory-based survey. PLoS ONE. 2013;8(3):e59373. 10.1371/journal.pone.0059373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Asmundsdo´ttir LR, Erlendsdo´ttir H, Gottfredsson M. Increasing incidence of candidemia: results from a 20-year nationwide study in Iceland. J Clin Microbiol. 2002;4:3489–92. 10.1128/JCM.40.9.3489-3492.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sendid B, Cotteau A, François N, d’Haveloose A, Standaert A, Camus D, Poulain D. Candidaemia and antifungal therapy in a French University Hospital: rough trends over a decade and possible links. BMC Infect Dis. 2006;6(1):1–9. 10.1186/1471-2334-6-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Celebi S, Hacimustafaoglu M, Ozdemir O, Ozkaya G. Nosocomial candidaemia in children: results of a 9-year study. Mycoses. 2008;51(3):248–57. 10.1111/j.1439-0507.2007.01464.x. [DOI] [PubMed] [Google Scholar]
- 9.Fortún J, Martín-Dávila P, de la Pedrosa EGG, Pintado V, Cobo J, Fresco G, Agundez M. Emerging trends in candidemia: a higher incidence but a similar outcome. J Infect. 2012;65(1):64–70. 10.1016/j.jinf.2012.02.011. [DOI] [PubMed] [Google Scholar]
- 10.Oud L. Secular trends in uitilization of critical care services among Candidemia-Associated hospitalizations: a Population-based Cohort Study. J Clin Med Res. 2015;8(1):40–3. 10.14740/jocmr2387w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Colombo AL, Nucci M, Park BJ, Nouér SA, Arthington-Skaggs B, da Matta DA, Morgan J. Epidemiology of candidemia in Brazil: a nationwide sentinel surveillance of candidemia in eleven medical centers. J Clin Microbiol. 2006;44(8):2816–23. 10.1128/JCM.00773-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Horn DL, Neofytos D, Anaissie EJ, Fishman JA, Steinbach WJ, et al. Epidemiology and outcomes of candidemia in 2019 patients: data from the prospective antifungal therapy alliance registry. Clin Infect Dis. 2009;48:1695–703. 10.1086/599039. [DOI] [PubMed] [Google Scholar]
- 13.Bassetti M, Taramasso L, Nicco E, Molinari MP, Mussap M, Viscoli C. Epidemiology, species distribution, antifungal susceptibility and outcome of nosocomial candidemia in a tertiary care hospital in Italy. PLoS ONE. 2011;6(9):e24198. 10.1371/journal.pone.0024198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zaoutis TE, Argon J, Chu J, Berlin JA, Walsh TJ, Feudtner C. The epidemiology and attributable outcomes of candidemia in adults and children hospitalized in the United States: a propensity analysis. Clin Infect Dis. 2005;41(9):1232–9. 10.1086/496922. [DOI] [PubMed] [Google Scholar]
- 15.Sipsas NV, Lewis RE, Tarrand J, Hachem R, Rolston KV, Raad II, Kontoyiannis DP. Candidemia in patients with hematologic malignancies in the era of new antifungal agents (2001-2007). Cancer. 2009;115(20):4745–52. 10.1002/cncr.24507. [DOI] [PubMed] [Google Scholar]
- 16.Sabino Z, Verissimo R, Brandao C, Alves J, Parada C, Rosado H, L., Pais C. Epidemiology of candidemia in oncology patients: a 6-year survey in a Portuguese central hospital. Med Mycol. 2010;48(2):346–54. 10.3109/13693780903161216. [DOI] [PubMed] [Google Scholar]
- 17.Bergamasco MD, Garnica M, Colombo AL, Nucci M. Epidemiology of candidemia in patients with hematologic malignancies and solid tumours in Brazil. Mycoses. 2013;56(3):256–63. 10.1111/myc.12013. [DOI] [PubMed] [Google Scholar]
- 18.Colombo AL, Guimarães T, Sukienik T, Pasqualotto AC, Andreotti R, Queiroz-Telles F, Nucci M. Prognostic factors and historical trends in the epidemiology of candidemia in critically ill patients: an analysis of five multicenter studies sequentially conducted over a 9-year period. Intensive Care Med. 2014;40(10):1489–98. 10.1007/s00134-014-3400-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Raza A, Zafar W, Mahboob A, Nizammudin S, Rashid N, Sultan F. Clinical features and outcomes of Candidaemia in cancer patients: results from Pakistan. J Pak Med Assoc. 2016;66(5):584–9. PMID: 27183941. [PubMed] [Google Scholar]
- 20.Bassetti M, Righi E, Ansaldi F, Merelli M, Cecilia T, De Pascale G, Trecarichi EM. A multicenter study of septic shock due to candidemia: outcomes and predictors of mortality. Intensive Care Med. 2014;40(6):839–45. 10.1007/s00134-014-3310-z. [DOI] [PubMed] [Google Scholar]
- 21.Ma CF, Li FQ, Shi LN, Hu YA, Wang Y, Huang M, Kong QQ. Surveillance study of species distribution, antifungal susceptibility and mortality of nosocomial candidemia in a tertiary care hospital in China. BMC Infect Dis. 2013;13(1):337. 10.1186/1471-2334-13-337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gokcebay DG, Yarali N, Isik P, Bayram C, Ozkaya-Parlakay A, Kara A, Tunç B. Candida associated bloodstream infections in pediatric hematology patients: a single center experience. Mediterranean J Hematol Infect Dis. 2016;8(1). 10.4084/MJHID.2016.018. [DOI] [PMC free article] [PubMed]
- 23.Slavin MA, Sorrell TC, Marriott D, Thursky KA, Nguyen Q, Ellis DH, Chen S. C. A. Candidaemia in adult cancer patients: risks for fluconazole-resistant isolates and death. J Antimicrob Chemother. 2010;65(5):1042–51. 10.1093/jac/dkq053. [DOI] [PubMed] [Google Scholar]
- 24.Lortholary O, Renaudat C, Sitbon K, Madec Y, Denoeud-Ndam L, Wolff M, French Mycosis Study Group. Worrisome trends in incidence and mortality of candidemia in intensive care units (Paris area, 2002–2010). Intensive Care Med. 2014;40(9):1303–12. 10.1007/s00134-014-3408-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.De Rosa FG, Trecarichi EM, Montrucchio C, Losito AR, Raviolo S, Posteraro B, Serra R. Mortality in patients with early-or late-onset candidaemia. Journal of Antimicrobial Chemotherapy. 2012; dks480. 10.1093/jac/dks480. [DOI] [PMC free article] [PubMed]
- 26.Li C, Wang H, Yin M, Han H, Yue JF, Zhang F, Wu DW. The differences in the epidemiology and predictors of death between candidemia acquired in intensive care units and other hospital settings. Intern Med. 2015;54(23):3009–16. 10.2169/internalmedicine.54.3744. [DOI] [PubMed] [Google Scholar]
- 27.Basu S, Gugnani HC, Joshi S, Gupta N. Distribution of Candida species in different clinical sources in Delhi, India, and proteinase and phospholipase activity of Candida albicans isolates. REVISTA IBEROAMERICANA DE MICOLOGIA. 2003;20(4):137–40. PMID: 15456350. [PubMed] [Google Scholar]
- 28.Shin JH, Kee SJ, Shin MG, Kim SH, Shin DH, Lee SK, Ryang DW. Biofilm production by isolates of Candida species recovered from nonneutropenic patients: comparison of bloodstream isolates with isolates from other sources. J Clin Microbiol. 2002;40(4):1244–8. 10.1128/jcm.40.4.1244-1248.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Růzicka F, Hola V, Votava M, Tejkalová R. Detection and significance of biofilm formation in yeasts isolated from hemocultures. Klinicka Mikrobiologie Infekcni Lekarstvi. 2006;12(4):150–5. PMID: 16958020. [PubMed] [Google Scholar]
- 30.Douglas LJ. Candida biofilms and their role in infection. Trends Microbiol. 2003;11(1):30–6. 10.1016/S0966-842X(02)00002-1. [DOI] [PubMed] [Google Scholar]
- 31.Kumamoto CA, Vinces MD. Alternative Candida albicans lifestyles: growth on surfaces. Annu Rev Microbiol. 2005;59:113–33. 10.1146/annurev.micro.59.030804.121034. [DOI] [PubMed] [Google Scholar]
- 32.Al-Fattani MA, Douglas LJ. Biofilm matrix of Candida albicans and Candida tropicalis: chemical composition and role in drug resistance. J Med Microbiol. 2006;55(8):999–1008. 10.1099/jmm.0.46569-0. [DOI] [PubMed] [Google Scholar]
- 33.Mukherjee PK, Chandra J, Kuhn DM, Ghannoum MA. Mechanism of fluconazole resistance in Candida albicans biofilms: phase-specific role of efflux pumps and membrane sterols. Infect Immun. 2003;71(8):4333–40. 10.1128/iai.71.8.4333-4340.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nett J, Lincoln L, Marchillo K, Massey R, Holoyda K, Hoff B, Andes D. Putative role of β-1, 3 glucans in Candida albicans biofilm resistance. Antimicrob Agents Chemother. 2007;51(2):510–20. 10.1128/aac.01056-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tsui C, Kong EF, Jabra-Rizk MA. Pathogenesis of Candida albicans biofilm. Pathogens Disease. 2016;74(4). 10.1093/femspd/ftw018. [DOI] [PMC free article] [PubMed]
- 36.Kojic EM, Darouiche RO. Candida infections of medical devices. Clin Microbiol Rev. 2004;17(2):255–67. 10.1128/cmr.17.2.255-267.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kumamoto CA. Candida biofilms. Curr Opin Microbiol. 2002;5(6):608–11. 10.1016/s1369-5274(02)00371-5. [DOI] [PubMed] [Google Scholar]
- 38.Kuhn DM, George T, Chandra J, Mukherjee PK, Ghannoum MA. Antifungal susceptibility of Candida biofilms: unique efficacy of amphotericin B lipid formulations and echinocandins. Antimicrob Agents Chemother. 2002;46(6):1773–80. 10.1128/aac.46.6.1773-1780.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Simitsopoulou M, Peshkova P, Tasina E, Katragkou A, Kyrpitzi D, Velegraki A, Roilides E. Species-specific and drug-specific differences in susceptibility of Candida biofilms to echinocandins: characterization of less common bloodstream isolates. Antimicrob Agents Chemother. 2013;57(6):2562–70. 10.1128/aac.02541-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Garnacho-Montero J, Díaz-Martín A, García-Cabrera E, de Pipaón MRP, Hernández-Caballero C, Lepe-Jiménez JA. Impact on hospital mortality of catheter removal and adequate antifungal therapy in Candida spp. bloodstream infections. J Antimicrob Chemother. 2013;68(1):206–13. 10.1093/jac/dks347. [DOI] [PubMed] [Google Scholar]
- 41.Andes DR, Safdar N, Baddley JW, Playford G, Reboli AC, Rex JH, Mycoses Study Group. Impact of treatment strategy on outcomes in patients with candidemia and other forms of invasive candidiasis: a patient-level quantitative review of randomized trials. Clin Infect Dis. 2012. 10.1093/cid/cis021. cis021. [DOI] [PubMed] [Google Scholar]
- 42.Bassetti M, Merelli M, Ansaldi F, De Florentiis D, Sartor A, Scarparo C, Righi E. Clinical and therapeutic aspects of candidemia: a five-year single centre study. PLoS ONE. 2015;10(5):e0127534. 10.1371/journal.pone.0127534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tedeschi S, Tumietto F, Giannella M, Bartoletti M, Cristini F, Cioni G, Viale P. Epidemiology and outcome of candidemia in internal medicine wards: a regional study in Italy. Eur J Intern Med. 2016;34:39–44. 10.1016/j.ejim.2016.08.020. [DOI] [PubMed] [Google Scholar]
- 44.Rajendran R, Sherry L, Nile CJ, Sherriff A, Johnson EM, Hanson MF, Ramage G. Biofilm formation is a risk factor for mortality in patients with Candida albicans bloodstream infection—Scotland, 2012–2013. Clin Microbiol Infect. 2016;22(1):87–93. 10.1016/j.cmi.2015.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tumbarello M, Fiori B, Trecarichi EM, Posteraro P, Losito AR, De Luca A, Posteraro B. Risk factors and outcomes of candidemia caused by biofilm-forming isolates in a tertiary care hospital. PLoS ONE. 2012;7(3). 10.1371/journal.pone.0033705. e33705. [DOI] [PMC free article] [PubMed]
- 46.Tumbarello M, Posteraro B, Trecarichi EM, Fiori B, Rossi M, Porta R, Cauda R. Biofilm production by Candida species and inadequate antifungal therapy as predictors of mortality for patients with candidemia. J Clin Microbiol. 2007;45(6):1843–50. 10.1128/jcm.00131-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tascini C, Sozio E, Corte L, Sbrana F, Scarparo C, Ripoli A, Bassetti M. The role of biofilm forming on mortality in patients with candidemia: a study derived from real world data. Infect Dis. 2018;50(3):214–9. 10.1080/23744235.2017.1384956. [DOI] [PubMed] [Google Scholar]
- 48.Vitális E, Nagy F, Tóth Z, Forgács L, Bozó A, Kardos G, Kovács R. Candida biofilm production is associated with higher mortality in patients with candidaemia. Mycoses. 2020;63(4):352–60. 10.1111/myc.13049. [DOI] [PubMed] [Google Scholar]
- 49.Guembe M, Guinea J, Marcos-Zambrano L, Fernández-Cruz A, Peláez T, Muñoz P, Bouza E. Is biofilm production a predictor of catheter-related candidemia? Med Mycol. 2014;52(4):407–10. 10.1093/mmy/myt031. [DOI] [PubMed] [Google Scholar]
- 50.Pongrácz J, Benedek K, Juhász E, Iván M, Kristóf K. In vitro biofilm production of Candida bloodstream isolates: any association with clinical characteristics? J Med Microbiol. 2016;65(4):272–7. 10.1099/jmm.0.000207. [DOI] [PubMed] [Google Scholar]
- 51.Muñoz P, Agnelli C, Guinea J, Vena A, Álvarez-Uría A, Marcos-Zambrano LJ, Bouza E. Is biofilm production a prognostic marker in adults with candidaemia? Clin Microbiol Infect. 2018;24(9):1010–5. 10.1016/j.cmi.2018.01.022. [DOI] [PubMed] [Google Scholar]
- 52.Riedel S, Eisinger SW, Dam L, Stamper PD, Carroll KC. Comparison of BD Bactec Plus Aerobic/F medium to VersaTREK Redox 1 blood culture medium for detection of Candida spp. in seeded blood culture specimens containing therapeutic levels of antifungal agents. J Clin Microbiol. 2011;49(4):1524–9. 10.1128/jcm.02260-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jekarl DW, Lee SY, Lee S, Park YJ, Lee J, Baek SM, Lee MK. Comparison of the Bactec Fx Plus, Mycosis IC/F, Mycosis/F lytic blood culture media and the BacT/Alert 3D FA media for detection of Candida species in seeded blood culture specimens containing therapeutic peak levels of fluconazole. J Clin Lab Anal. 2012;26(6):412–9. 10.1002/jcla.21535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Viganò EF, Vasconi E, Agrappi C, Clerici P. Use of simulated blood cultures for time to detection comparison between BacT/ALERT™ and BACTEC™ 9240 blood culture systems. Diagn Microbiol Infect Dis. 2002;44(3):235–40. 10.1016/S0732-8893(02)00451-0. [DOI] [PubMed] [Google Scholar]
- 55.McGinnis MR. Laboratory handbook of medical mycology. Elsevier. 2012. ISBN: 9780124828506.
- 56.Odds FC, Bernaerts RIA, CHROMagar. Candida, a new differential isolation medium for presumptive identification of clinically important Candida species. J Clin Microbiol. 1994;32(8):1923–9. 10.1128/jcm.32.8.1923-1929.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Buesching WJ, Kurek K, Roberts GD. Evaluation of the modified API 20 C system for identification of clinically important yeasts. J Clin Microbiol. 1979;9(5):565–9. 10.1128/jcm.9.5.565-569.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Land GA, Harrison BA, Hulme KL, Cooper BH, Byrd JC. Evaluation of the new API 20 C strip for yeast identification against a conventional method. J Clin Microbiol. 1979;10(3):357–64. 10.1128/jcm.10.3.357-364.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Pfaller MA, Diekema DJ, Messer SA, Boyken L, Hollis RJ. Activities of fluconazole and voriconazole against 1,586 recent clinical isolates of Candida species determined by broth microdilution, disk diffusion, and Etest methods: report from the ARTEMIS Global Antifungal susceptibility program, 2001. J Clin Microbiol. 2003;41(4):1440–6. 10.1128/jcm.41.4.1440-1446.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Barry AL, Pfaller MA, Rennie RP, Fuchs PC, Brown SD. Precision and accuracy of fluconazole susceptibility testing by broth microdilution, Etest, and disk diffusion methods. Antimicrob Agents Chemother. 2002;46(6):1781–4. 10.1128/aac.46.6.1781-1784.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hawser SP, Norris H, Jessup CJ, Ghannoum M. Comparison of a 2, 3-bis (2-methoxy-4-nitro-5-sulfophenyl)-5-[(phenylamino) carbonyl]-2 H-tetrazolium hydroxide (XTT) colorimetric method with the standardized National Committee for Clinical Laboratory Standards method of testing clinical yeast isolates for susceptibility to antifungal agents. J Clin Microbiol. 1998;36(5):1450–2. 10.1128/jcm.36.5.1450-1452.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kuhn DM, Balkis M, Chandra J, Mukherjee PK, Ghannoum MA. Uses and limitations of the XTT assay in studies of Candida growth and metabolism. J Clin Microbiol. 2003;41(1):506–8. 10.1128/jcm.41.1.506-508.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Malchikova A, Klyasova G. In vitro activity of anidulafungin, caspofungin, fluconazole and amphotericin B against biofilms and planktonic forms of Candida species isolated from blood culture in patients with hematological malignancies. J Med Mycol. 2021;31(3):101162. 10.1016/j.mycmed.2021.101162. [DOI] [PubMed] [Google Scholar]
- 64.Hasan F, Xess I, Wang X, Jain N, Fries BC. Biofilm formation in clinical Candida isolates and its association with virulence. Microbes Infect. 2009;11(8–9):753–61. 10.1016/j.micinf.2009.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Liu CY, Huang LJ, Wang WS, Chen TL, Yen CC, Yang MH, Chiou TJ. Candidemia in cancer patients: impact of early removal of non-tunneled central venous catheters on outcome. J Infect. 2009;58(2):154–60. 10.1016/j.jinf.2008.12.008. [DOI] [PubMed] [Google Scholar]
- 66.Kleinbaum DG, Klein M. The stratified Cox procedure. Survival analysis. New York, NY: Springer; 2012. pp. 201–40. 10.1007/978-1-4419-6646-9_5. [Google Scholar]
- 67.Suh JW, Kim MJ, Kim JH. Risk factors of septic shock development and thirty-day mortality with a predictive model in adult candidemia patients in intensive care units. Infect Dis. 2021;53(12):908–19. 10.1080/23744235.2021.1959052. [DOI] [PubMed] [Google Scholar]
- 68.Moon IK, Lee EJ, Kang HC, Yu SN, Wee JW, Kim TH, Park SY. Risk factors for mortality in patients with candidemia and the usefulness of a Candida Score. Korean J Med Mycol. 2013;18(3):59–65. [Google Scholar]
- 69.Almirante B, Rodríguez D, Park BJ, Cuenca-Estrella M, Planes AM, Almela M, Pahissa A. Epidemiology and predictors of mortality in cases of Candida bloodstream infection: results from population-based surveillance, Barcelona, Spain, from 2002 to 2003. J Clin Microbiol. 2005;43(4):1829–35. 10.1128/JCM.43.4.1829-1835.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Murri R, Scoppettuolo G, Ventura G, Fabbiani M, Giovannenze F, Taccari F, Fantoni M. Initial antifungal strategy does not correlate with mortality in patients with candidemia. Eur J Clin Microbiol Infect Dis. 2016;35(2):187–93. 10.1007/s10096-015-2527-2. [DOI] [PubMed] [Google Scholar]
- 71.Takuma T, Shoji H, Niki Y. Terminal-stage prognostic analysis in candidemia. J Infect Chemother. 2015;21(5):376–80. 10.1016/j.jiac.2015.01.007. [DOI] [PubMed] [Google Scholar]
- 72.Stergiopoulou T, Meletiadis J, Sein T, Papaioannidou P, Tsiouris I, Roilides E, et al. Comparative pharmacodynamic interaction analysis between ciprofloxacin, moxifloxacin and levofloxacin and antifungal agents against Candida albicans and Aspergillus Fumigatus. J Antimicrob Chemother. 2009;63:343–8. 10.1093/jac/dkn473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ala-Houhala M, Valkonen M, Kolho E, Friberg N, Anttila VJ. Clinical and microbiological factors associated with mortality in candidemia in adult patients 2007–2016. Infect Dis. 2019;51(11–12):824–30. 10.1080/23744235.2019.1662941. [DOI] [PubMed] [Google Scholar]
- 74.Falagas ME, Apostolou KE, Pappas VD. Attributable mortality of candidemia: a systematic review of matched cohort and case-control studies. Eur J Clin Microbiol Infect Dis. 2006;25(7):419–25. 10.1007/s10096-006-0159-2. [DOI] [PubMed] [Google Scholar]
- 75.Velasco E, Bigni R. A prospective cohort study evaluating the prognostic impact of clinical characteristics and comorbid conditions of hospitalized adult and pediatric cancer patients with candidemia. Eur J Clin Microbiol Infect Dis. 2008;27(11):1071–8. 10.1007/s10096-008-0546-y. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data is provided within the manuscript or supplementary information files.