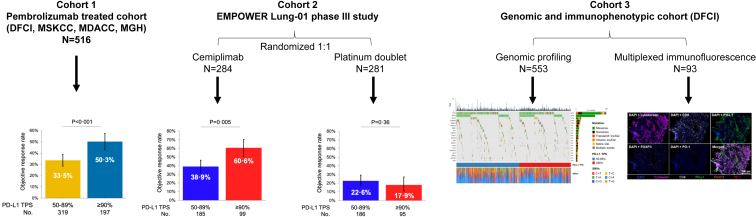
Figure 1.
Study schema. Patients with metastatic NSCLC and a PD-L1 TPS greater than or equal to 50% who were treated with first-line pembrolizumab at DFCI, MSKCC, MDACC, and MGH were included in cohort 1. Patients who enrolled in the randomized, phase III trial EMPOWER-Lung 01 of first-line cemiplimab versus chemotherapy were included in cohort 2 (independent validation cohort). Cohort 3 included a separate subset of patients with NSCLC who underwent full tumor genomic profiling and multiplexed immunofluorescence at DFCI and had matched PD-L1 expression assessment. This cohort was used to determine the genomic and immunophenotypic correlates of very high and high PD-L1 TPS. DFCI, Dana-Farber Cancer Institute; MDACC, MD Anderson Cancer Center; MGH, Massachusetts General Hospital; MSKCC, Memorial Sloan Kettering Cancer Center; PD-L1, programmed death-ligand 1; TPS, tumor proportion score.