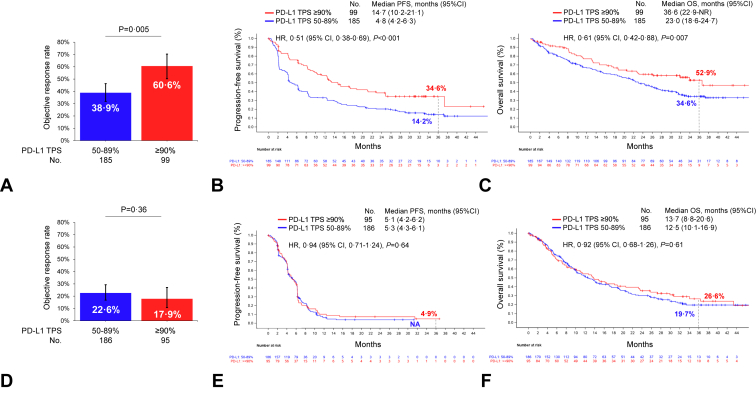
Figure 3.
(A) Objective response rate, (B) three-year progression-free survival rates, and (C) three-year overall survival rates to first-line cemiplimab in patients with advanced NSCLC and PD-L1 TPS of greater than or equal to 90% versus 50% to 89% in the EMPOWER-Lung 1 study. (D) Objective response rate, (E) three-year progression-free survival rates, and (F) three-year overall survival rates to first-line chemotherapy in patients with advanced NSCLC and PD-L1 TPS of greater than or equal to 90% versus 50% to 89% in the EMPOWER-Lung 1 study. CI, confidence interval; HR, hazard ratio; OS, overall survival; PD-L1, programmed death-ligand 1; TPS, tumor proportion score.