Dear Sir:
Endovascular thrombectomy (EVT) is the standard of care for acute stroke, wherein achieving complete recanalization may improve clinical outcomes [1]. However, fragile thrombi are susceptible to fragmentation during EVT, causing distal embolization and migration, which diminishes the likelihood of complete recanalization [2-5]. Thrombus fragility may be predominantly affected by its structure and composition. In this study, we investigated the association between thrombus fragility and tumor histology.
This study included consecutive patients who underwent successful thrombus retrieval by EVT between January 2016 and December 2021, had their retrieved thrombi histologically evaluated, and were enrolled in the prospective registry. Eligible participants were patients primarily treated with a stent retriever; we excluded those who underwent only contact aspiration thrombectomy. This study was approved by the Institutional Review Board of Severance Hospital (4-2023-1010). Written informed consent was obtained from the patients or their next of kin for enrollment in the cohort and for utilizing the retrieved thrombi in the study.
EVT was performed under local anesthesia, according to common recommendations (Supplementary Methods). The retrieved thrombi were immediately fixed in 4% paraformaldehyde. All thrombi were immunohistochemically stained for erythrocytes, platelets, fibrin, lymphocytes, neutrophils, monocytes, tissue factors, and neutrophil extracellular traps (Supplementary Methods and Supplementary Table 1) [6]. Images of stained thrombi were acquired using a whole-slide scanner or Stereo Investigator Imaging system (MBF Bioscience, Williston, VT, USA) equipped with a light microscope (Axio Imager D2; Carl Zeiss Co., Ltd., Jena, Germany). The acquired images were analyzed using an Automated Region-of-interest-based Image Analysis software (Supplementary Methods) [7]. Each thrombus composition fraction (%) was determined by calculating the pixel density as a percentage of the total thrombus area.
All conventional angiographic images obtained immediately after each thrombectomy attempt were reviewed. The presence of a fragile thrombus was defined as either the thrombus fragmentation during EVT or the presence of initially multiple intracranial occlusions. Thrombus fragmentation manifests as downstream occlusion subsequent to thrombectomy attempts, characterized by angiographical occlusion distal to the original target site coupled with recanalization of the initial target [2-4]. Furthermore, the presence of initially multiple intracranial occlusions was considered indicative of a fragile thrombus, as it may reflect pre-existing fragmentation during thrombus growth or embolism. Two independent neurointerventionalists (JHB and BMK), blinded to the histological and clinical information, assessed the images (κ-values: 0.79 for thrombus fragmentation, 0.82 for successful recanalization, and 0.91 for first-pass effect). Discrepancies were resolved through consensus.
In a cohort of 376 patients who underwent EVT and had their thrombi retrieved, immunohistochemistry was performed on 340 (90.4%) (Supplementary Figure 1). Finally, 310 patients were included in the study (mean age, 70.9±13.6 years; men, 49.4%) (Table 1). Fragile thrombi were observed in 115 (37.1%) patients. Atrial fibrillation (67.8% vs. 51.3%; P=0.004) and internal carotid artery (ICA) occlusion (40.9% vs. 19.0%; P<0.001) were more frequent in patients with fragile thrombi than in those without (Table 1). Regarding thrombus composition, fragile thrombi were significantly associated with higher fractions of erythrocytes (40.2% [30.7–51.4] vs. 31.0% [16.6–43.3]; P<0.001) (Table 1). A higher fraction of erythrocytes relative to whole common thrombus components (i.e., erythrocytes, platelets, fibrin, and leukocytes) was also significantly associated with a fragile thrombus (44.0% [35.9–54.2] vs. 33.3% [20.2–42.3]; P<0.001). Multivariable analysis identified ICA occlusion (adjusted odds ratio, 2.45; 95% confidence interval, 1.41–4.27; P=0.001) and erythrocyte composition in thrombus (adjusted odds ratio, 1.14 per 5% composition; 95% confidence interval, 1.05–1.23; P<0.001) as independent factors for fragile thrombus (Table 2 and Figure 1). Erythrocyte composition in the thrombus could predict fragile thrombus (area under the receiver operating characteristic curve, 0.655; cutoff, 26.9%; sensitivity, 84.4%; specificity, 42.1%; P<0.001) (Supplementary Figure 2). Platelet composition tended to be lower in a fragile thrombus (8.9% [3.8–17.4] vs. 10.9 [4.5–20.7]; P=0.057).
Table 1.
Comparison of clinical and histological findings according to thrombus fragility
| Total (n=310) | Fragile thrombus (n=115) | No fragile thrombus (n=195) | P | |
|---|---|---|---|---|
| Demographics and stroke risk factors | ||||
| Age (yr) | 70.9±13.6 | 70.8±13.7 | 70.9±13.6 | 0.951 |
| Male sex | 153 (49.4) | 57 (49.6) | 96 (49.2) | 0.955 |
| Hypertension | 224 (72.3) | 80 (69.6) | 144 (73.8) | 0.416 |
| Diabetes | 100 (32.3) | 34 (29.6) | 66 (33.8) | 0.436 |
| Dyslipidemia | 81 (26.1) | 24 (20.9) | 57 (29.2) | 0.106 |
| Current smoking | 40 (12.9) | 15 (13.0) | 25 (12.8) | 0.955 |
| Coronary artery occlusive disease | 46 (14.8) | 14 (12.2) | 32 (16.4) | 0.311 |
| Atrial fibrillation | 178 (57.4) | 78 (67.8) | 100 (51.3) | 0.004 |
| Clinical conditions | ||||
| Initial NIHSS score | 14.0 [9.0–18.0] | 15.0 [10.0–18.0] | 12.0 [8.0–18.0] | 0.089 |
| Intravenous tPA administration | 114 (36.8) | 47 (40.9) | 67 (34.4) | 0.251 |
| Location of occlusions | <0.001 | |||
| Internal carotid artery | 84 (27.1) | 47 (40.9) | 37 (19.0) | |
| M1 segment of middle cerebral artery | 120 (38.7) | 41 (35.7) | 79 (40.5) | |
| M2 segment of middle cerebral artery | 75 (24.2) | 21 (18.3) | 54 (27.7) | |
| Anterior cerebral artery | 1 (0.3) | 0 (0.0) | 1 (0.5) | |
| Vertebrobasilar artery | 26 (8.4) | 4 (3.4) | 22 (11.3) | |
| Posterior cerebral artery | 4 (1.3) | 2 (1.7) | 2 (1.0) | |
| Tandem occlusion | 26 (8.4) | 10 (8.7) | 16 (8.2) | 0.880 |
| Onset-to-puncture time (min) | 292.0 [167.0–610.0] | 267.0 [138.0–571.0] | 308.0 [176.0–624.0] | 0.210 |
| Use of balloon guide catheter* | 278 (89.7) | 108 (99.1) | 170 (99.4) | 0.747 |
| Endovascular outcomes | ||||
| Recanalization status† | ||||
| Successful recanalization | 287 (92.6) | 104 (90.4) | 183 (93.8) | 0.268 |
| Complete recanalization | 193 (62.3) | 37 (32.2) | 156 (80.0) | <0.001 |
| First-pass effect | 102 (32.9) | 5 (4.4) | 97 (49.7) | <0.001 |
| Number of passes of stent retriever | 2.3±1.4 | 2.8±1.3 | 2.0±1.4 | <0.001 |
| Puncture-to-recanalization time (min) | 31.0 [20.0–49.5] | 33.5 [24.0–53.0] | 29.0 [19.0–49.0] | 0.128 |
| Thrombus composition (%) | ||||
| Erythrocyte | 35.6 [20.3–46.7] | 40.2 [30.7–51.4] | 31.0 [16.6–43.3] | <0.001 |
| Platelet | 9.7 [4.3–19.5] | 8.9 [3.8–17.4] | 10.9 [4.5–20.7] | 0.057 |
| Fibrin | 32.3 [21.2–47.2] | 31.6 [21.0–48.1] | 32.7 [21.4–45.7] | 0.875 |
| Leukocytes | 8.5 [4.2–15.7] | |||
| Lymphocyte | 0.3 [0.2–0.5] | 0.3 [0.2–0.5] | 0.2 [0.1–0.4] | 0.135 |
| Neutrophil | 0.6 [0.2–2.1] | 0.7 [0.2–2.5] | 0.6 [0.2–2.0] | 0.813 |
| Monocyte | 5.0 [2.2–11.1] | 5.1 [2.3–10.7] | 4.9 [2.1–11.6] | 0.989 |
| Tissue factor | 12.8 [1.5–42.7] | 13.9 [1.9–45.1] | 11.8 [1.1–41.0] | 0.608 |
| Neutrophil extracellular trap | 2.7 [1.1–5.1] | 3.0 [1.1–6.4] | 2.7 [1.1–4.6] | 0.368 |
Values represent mean±standard deviation, number (%), or median [interquartile range].
NIHSS, National Institutes of Health Stroke Scale; tPA, tissue-type plasminogen activator.
The calculation of the percentages of patients using a balloon-guided catheter was limited to those with anterior circulation stroke;
Successful recanalization was defined as achieving final modified Thrombolysis In Cerebral Infarction (mTICI) 2b or 3; Complete recanalization was defined as the attainment of a final mTICI score of 3.
Table 2.
Clinical and histological factors associated with fragile thrombus
| Adjusted odds ratio (95% CI) | P | |
|---|---|---|
| Clinical factors | ||
| Atrial fibrillation | 1.61 (0.95–2.73) | 0.075 |
| Initial NIHSS score | 1.01 (0.97–1.06) | 0.494 |
| Internal carotid artery occlusion | 2.45 (1.41–4.27) | 0.001 |
| Histological factors | ||
| Erythrocyte, per 5% | 1.14 (1.05–1.23) | <0.001 |
| Platelet, per 5% | 0.99 (0.90–1.07) | 0.725 |
The analysis included variables with P<0.1 in the univariable analyses.
CI, confidence interval; NIHSS, National Institutes of Health Stroke Scale.
Figure 1.
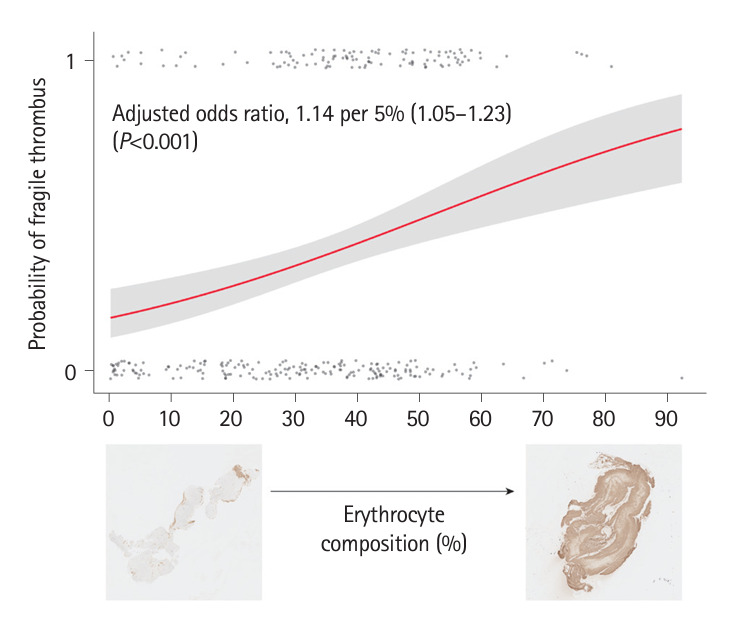
Probability of a fragile thrombus by erythrocyte composition.
In this study, a fragile thrombus was observed in 37.1% of cases, a frequency comparable to 35.2%–57.5%, as stated in previous studies employing similar definitions to ours [2,3]. It was also associated with ICA occlusion and erythrocyte-rich thrombi. ICA occlusion has been suggested to correlate with distal and secondary embolisms [2]. Preclinical studies have shown that erythrocyte-rich thrombi are softer, less stiff, and more prone to fracture, suggesting their fragility [8,9]. Experimental retrieval of erythrocyte-rich thrombi resulted in the generation of more embolic fragments [9]. These findings support the association between fragility and erythrocyte-rich thrombi observed in our study.
Erythrocytes, fibrin, and platelets exhibit different degrees of stability. Platelets aggregate with each other by adhesively binding integrin α2bβ3 to fibrinogen. Consequently, platelets are tightly attached to each other. In contrast, erythrocytes primarily aggregate via passive packing, making them more susceptible to external forces. This may explain why erythrocyte-rich thrombi were more fragile, whereas those with higher platelet content tended to be less fragile.
However, previous clinical studies have reported inconsistent results. While one study demonstrated an association between clot migration and higher erythrocyte content [5], others found associations of secondary embolism with high fibrin and low erythrocyte contents [4], as well as an association between thrombus fragmentation and higher lymphocyte counts [10]. Such disparities may arise from the varying definitions of fragile thrombi and smaller sample sizes in previous studies. Additionally, previous studies utilized hematoxylin-eosin and histochemical stains, such as Martius Scarlet Blue, to detect erythrocytes and fibrin; this study employed immunohistochemistry. Precise differentiation between the major thrombus components may occasionally be challenging with histochemical staining.
Despite our strengths, such as a relatively large sample size, precise analysis of thrombus components through immunohistochemistry, and a semi-automated software program, our study has limitations. First, it focused on patients who underwent EVT primarily using stent retrievers. This was because patients who underwent only contact aspiration thrombectomy were excluded from this study, potentially having different mechanical impacts on thrombus. Second, although thrombus fragmentation may not always result in downstream occlusion, potentially leading to embolism in a new territory, such occurrences are rare and unlikely to significantly impact the outcome of the study. Finally, the definition of a fragile thrombus in this study may not accurately identify all such cases.
In conclusion, we found that thrombi with a higher erythrocyte composition were more fragile.
Footnotes
Funding statement
This study was supported by a National Research Foundation of Korea (NRF) grant, funded by the Korean government (RNF-2021R1A2C2003658), and a grant from the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Republic of Korea (RS-2023-00265497).
Conflicts of interest
The authors have no financial conflicts of interest.
Author contribution
Conceptualization: JHB, JHH. Study design: JHB, JHH. Methodology: JHB, IK, JHH. Data collection: all authors. Investigation: all authors. Statistical analysis: JHB. Writing—original draft: JHB. Writing—review & editing: JHB, JHH. Funding acquisition: YDK, JHH. Approval of final manuscript: all authors.
Supplementary materials
Supplementary materials related to this article can be found online at https://doi.org/10.5853/jos.2024.00787.
Primary antibodies for histological components of thrombus
Patient selection flowchart.
Receiver operating characteristic curves of erythrocyte composition in the thrombus and internal carotid artery (ICA) occlusion to predict a fragile thrombus. The erythrocyte composition in the thrombus could predict a fragile thrombus (area under the receiver operating characteristic curve [AUC], 0.655; cutoff, 26.9%; sensitivity, 84.4%; specificity, 42.1%; P<0.001). ICA occlusion could also predict fragile thrombi (AUC, 0.609; sensitivity, 40.9%; specificity, 81.0%; P<0.001). Considering both erythrocyte composition and ICA occlusion, it could predict fragile thrombi (AUC, 0.642; sensitivity, 39.1%; specificity, 89.2%; P<0.001).
References
- 1.Aroor SR, Asif KS, Potter-Vig J, Sharma A, Menon BK, Inoa V, et al. Mechanical thrombectomy access for all? Challenges in increasing endovascular treatment for acute ischemic stroke in the United States. J Stroke. 2022;24:41–48. doi: 10.5853/jos.2021.03909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ye G, Qi P, Chen K, Tan T, Cao R, Chen J, et al. Risk of secondary embolism events during mechanical thrombectomy for acute ischemic stroke: a single-center study based on histological analysis. Clin Neurol Neurosurg. 2020;193:105749. doi: 10.1016/j.clineuro.2020.105749. [DOI] [PubMed] [Google Scholar]
- 3.Kaesmacher J, Boeckh-Behrens T, Simon S, Maegerlein C, Kleine JF, Zimmer C, et al. Risk of thrombus fragmentation during endovascular stroke treatment. AJNR Am J Neuroradiol. 2017;38:991–998. doi: 10.3174/ajnr.A5105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sporns PB, Hanning U, Schwindt W, Velasco A, Buerke B, Cnyrim C, et al. Ischemic stroke: histological thrombus composition and pre-interventional CT attenuation are associated with intervention time and rate of secondary embolism. Cerebrovasc Dis. 2017;44:344–350. doi: 10.1159/000481578. [DOI] [PubMed] [Google Scholar]
- 5.Sporns PB, Jeibmann A, Minnerup J, Broocks G, Nawabi J, Schön G, et al. Histological clot composition is associated with preinterventional clot migration in acute stroke patients. Stroke. 2019;50:2065–2071. doi: 10.1161/STROKEAHA.118.023314. [DOI] [PubMed] [Google Scholar]
- 6.Yoo J, Kwon I, Kim S, Kim HM, Kim YD, Nam HS, et al. Coagulation factor expression and composition of arterial thrombi in cancer-associated stroke. Stroke. 2023;54:2981–2989. doi: 10.1161/STROKEAHA.123.044910. [DOI] [PubMed] [Google Scholar]
- 7.Heo J, Seog Y, Lee H, Lee IH, Kim S, Baek JH, et al. Automated composition analysis of thrombus from endovascular treatment in acute ischemic stroke using computer vision. J Stroke. 2022;24:433–435. doi: 10.5853/jos.2022.02054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Boodt N, Snouckaert van Schauburg PRW, Hund HM, Fereidoonnezhad B, McGarry JP, Akyildiz AC, et al. Mechanical characterization of thrombi retrieved with endovascular thrombectomy in patients with acute ischemic stroke. Stroke. 2021;52:2510–2517. doi: 10.1161/STROKEAHA.120.033527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fereidoonnezhad B, Dwivedi A, Johnson S, McCarthy R, McGarry P. Blood clot fracture properties are dependent on red blood cell and fibrin content. Acta Biomater. 2021;127:213–228. doi: 10.1016/j.actbio.2021.03.052. [DOI] [PubMed] [Google Scholar]
- 10.Goebel J, Gaida BJ, Wanke I, Kleinschnitz C, Koehrmann M, Forsting M, et al. Is histologic thrombus composition in acute stroke linked to stroke etiology or to interventional parameters? AJNR Am J Neuroradiol. 2020;41:650–657. doi: 10.3174/ajnr.A6467. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Primary antibodies for histological components of thrombus
Patient selection flowchart.
Receiver operating characteristic curves of erythrocyte composition in the thrombus and internal carotid artery (ICA) occlusion to predict a fragile thrombus. The erythrocyte composition in the thrombus could predict a fragile thrombus (area under the receiver operating characteristic curve [AUC], 0.655; cutoff, 26.9%; sensitivity, 84.4%; specificity, 42.1%; P<0.001). ICA occlusion could also predict fragile thrombi (AUC, 0.609; sensitivity, 40.9%; specificity, 81.0%; P<0.001). Considering both erythrocyte composition and ICA occlusion, it could predict fragile thrombi (AUC, 0.642; sensitivity, 39.1%; specificity, 89.2%; P<0.001).


