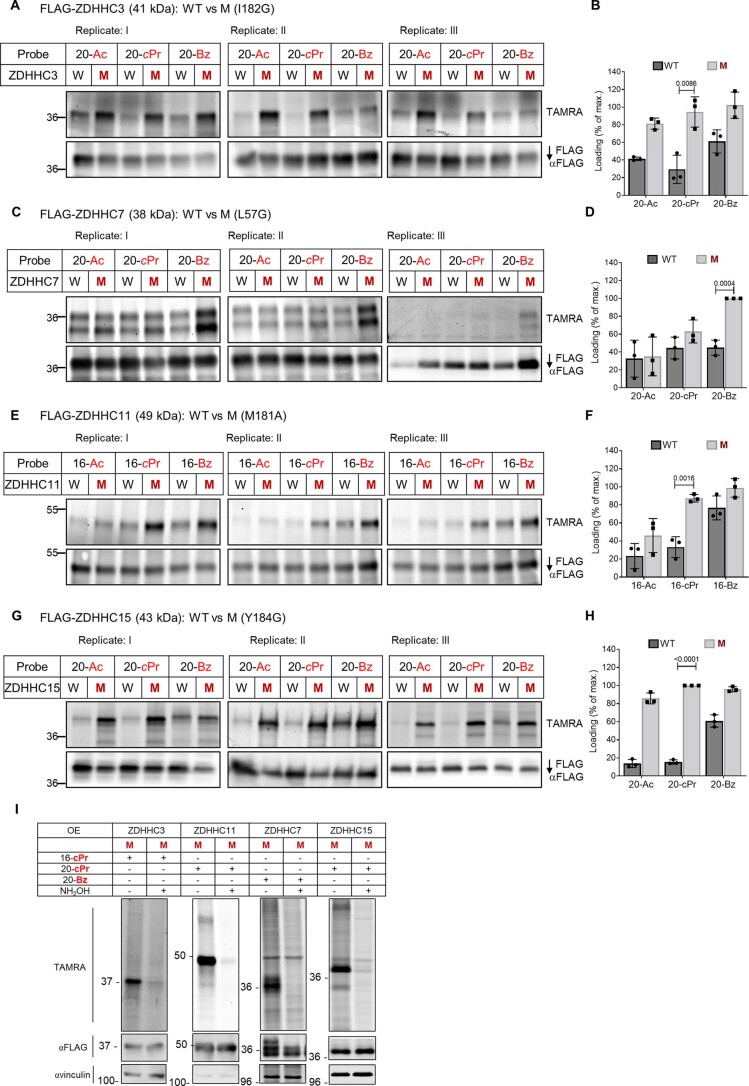
Extended Data Fig. 9. Bump optimization for mutants of ZDHHCs 3, 7, 11 and 15.
(a-h) WT (W in tables) and the indicated mutants of N-FLAG-tagged ZDHHC family members, ZDHHC3 (a-b), ZDHHC7 (c-d), ZDHHC11 (e-f), and ZDHHC15 (g-h), were subjected to loading assays with probes containing optimal chain length and Ac, cPr and Bz bump groups. WT and mutant constructs were transiently transfected into HEK293T cells and treated with 15 µM probe for 4 h. After cell lysis, constructs were immunoprecipitated on anti-FLAG resin, clicked with TAMRA-azide and separated by SDS-PAGE. Loading and input were visualized by in-gel fluorescence and anti-FLAG immunoblot, respectively. The average (n = 3 independent biological replicates) loading (b, d, f, and h) was reported as a percent of the maximal fluorescent: input ratios ± S.D. The two tailed unpaired t-test of Prism 9.0 was used to determine p-values and noted above relevant comparisons. (i) Thioester dependence of zDHHC7, zDHHC15, zDHHC3 and zDHHC11 labeling with bumped probes. HEK293T cells transiently expressing the acyltransferase mutants (M) were treated with C20-Bz (ZDHHC7), C20-cPr (ZDHHC15, ZDHHC11) or C16-cPr (ZDHHC3). Following CuAAC with TAMRA azide, lysates were treated with or without 0.8 M neutralized NH2OH. Representative images of 3 biological replicates (n = 3).