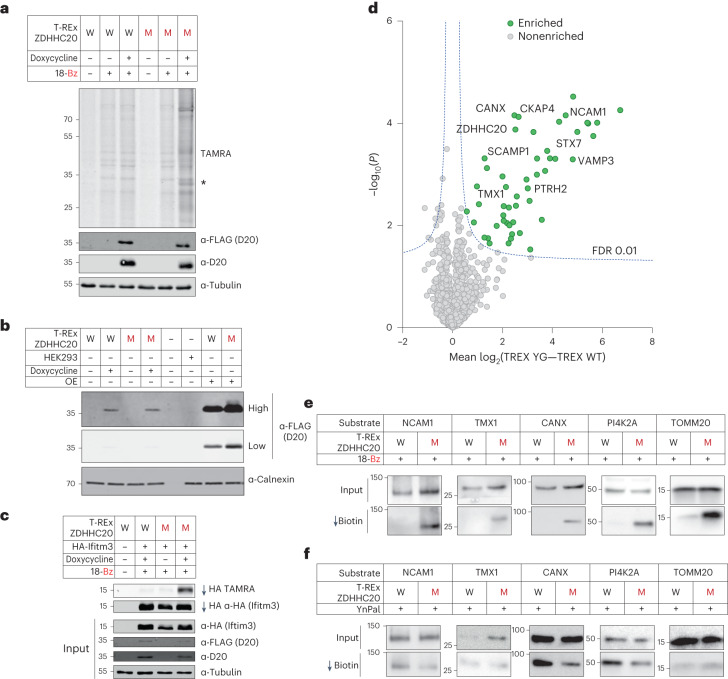
Fig. 5. Chemical–genetic analysis under inducible low-expression of ZDHHC20(Y181G).
a, Profile of WT ZDHHC20 (W) or ZDHHC20(Y181G) Flp-In 293 T-REx cell lines treated with 18-Bz (15 µM, 24 h). Lysates were clicked with TAMRA azide and then analyzed by in-gel fluorescence and SDS–PAGE. Note that the asterisk represents YG-dependent labeling of substrate protein bands. b, Comparison of protein expression levels between doxycycline induction of Flp-In 293 T-REx cells and overexpression by transient expression in HEK293T cells. Representative immunoblots are shown for FLAG at high or low exposure, to probe for ZDHHC20 WT versus ZDHHC20(Y181G), and calnexin as loading control (n = 3 independent biological replicates). c, In Flp-In 293 T-REx cells ZDHHC20(Y181G) retains exquisite selectivity for its substrate IFITM3 with the 18-Bz bumped probe, as seen in prior experiments. d, Chemical proteomic analysis of ZDHHC20 substrates in Flp-In 293 T-REx cells (15 µM 18-Bz, 24 h). Enrichment in T-REx ZDHHC20(Y181G) cells over T-REx WT ZDHHC20 reveals selective ZDHHC20 modification of substrates (green) (Student’s two-tailed unpaired t test, S0 = 0.5, adjusted FDR = 0.01, n = 4 independent biological replicates per condition). e,f, Validation of S-acylation for T-REx ZDHHC20(Y181G) substrates at endogenous levels. Flp-In 293 T-REx cells, WT ZDHHC20 (W) or ZDHHC20(Y181G) (M), induced with doxycycline for 24 h, were treated with 15 µM 18-Bz (e) or YnPal (f) for 24 h. Lysates were clicked with biotin azide before enrichment on neutravidin magnetic beads. Representative immunoblots are shown for input and pull-down signals (n = 2 independent replicates).