Abstract
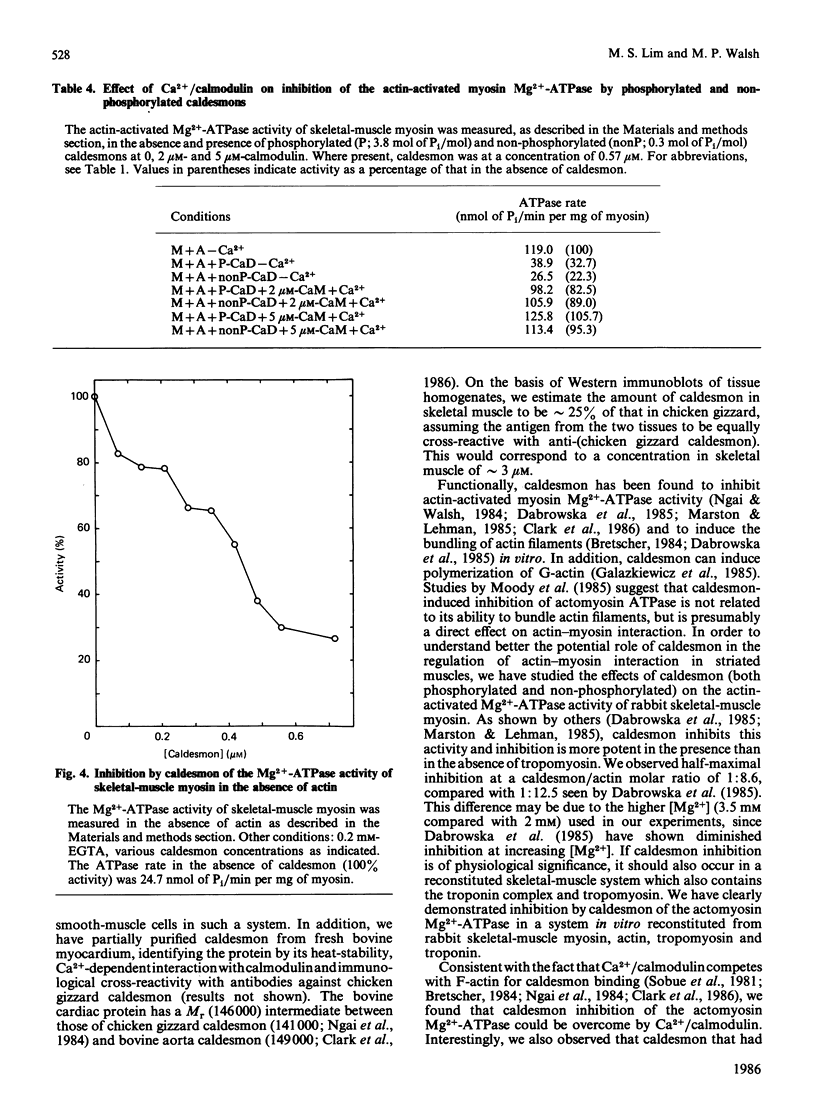
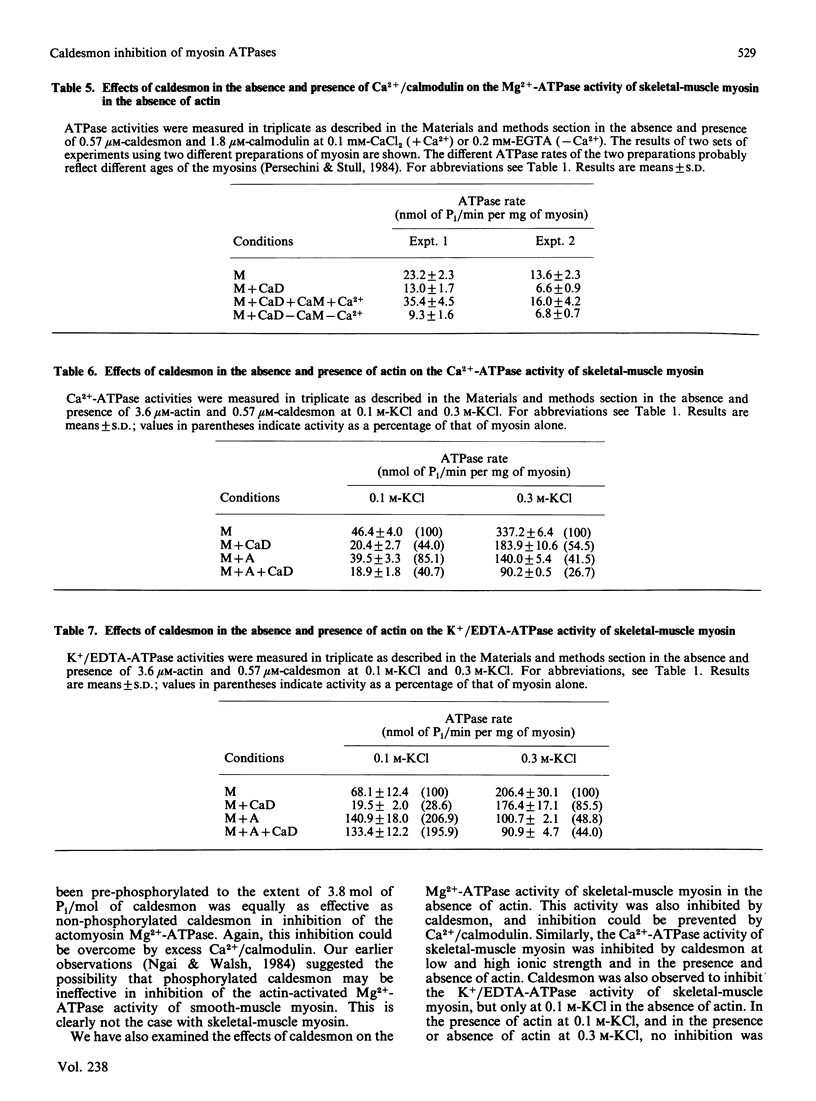
We studied the effects of caldesmon, a major actin- and calmodulin-binding protein found in a variety of muscle and non-muscle tissues, on the various ATPase activities of skeletal-muscle myosin. Caldesmon inhibited the actin-activated myosin Mg2+-ATPase, and this inhibition was enhanced by tropomyosin. In the presence of the troponin complex and tropomyosin, caldesmon inhibited the Ca2+-dependent actomyosin Mg2+-ATPase; this inhibition could be partly overcome by Ca2+/calmodulin. Caldesmon, phosphorylated to the extent of approximately 4 mol of Pi/mol of caldesmon, inhibited the actin-activated myosin Mg2+-ATPase to the same extent as did non-phosphorylated caldesmon. Both inhibitions could be overcome by Ca2+/calmodulin. Caldesmon also inhibited the Mg2+-ATPase activity of skeletal-muscle myosin in the absence of actin; this inhibition also could be overcome by Ca2+/calmodulin. Caldesmon inhibited the Ca2+-ATPase activity of skeletal-muscle myosin in the presence or absence of actin, at both low (0.1 M-KCl) and high (0.3 M-KCl) ionic strength. Finally, caldesmon inhibited the skeletal-muscle myosin K+/EDTA-ATPase at 0.1 M-KCl, but not at 0.3 M-KCl. Addition of actin resulted in no inhibition of this ATPase by caldesmon at either 0.1 M- or 0.3 M-KCl. These observations suggest that caldesmon may function in the regulation of actin-myosin interactions in striated muscle and thereby modulate the contractile state of the muscle. The demonstration that caldesmon inhibits a variety of myosin ATPase activities in the absence of actin indicates a direct effect of caldesmon on myosin. The inhibition of the actin-activated Mg2+-ATPase activity of myosin (the physiological activity) may not be due therefore simply to the binding of caldesmon to the actin filament causing blockage of myosin-cross-bridge-actin interaction.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bretscher A., Lynch W. Identification and localization of immunoreactive forms of caldesmon in smooth and nonmuscle cells: a comparison with the distributions of tropomyosin and alpha-actinin. J Cell Biol. 1985 May;100(5):1656–1663. doi: 10.1083/jcb.100.5.1656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bretscher A. Smooth muscle caldesmon. Rapid purification and F-actin cross-linking properties. J Biol Chem. 1984 Oct 25;259(20):12873–12880. [PubMed] [Google Scholar]
- Dabrowska R., Goch A., Gałazkiewicz B., Osińska H. The influence of caldesmon on ATPase activity of the skeletal muscle actomyosin and bundling of actin filaments. Biochim Biophys Acta. 1985 Sep 27;842(1):70–75. doi: 10.1016/0304-4165(85)90295-8. [DOI] [PubMed] [Google Scholar]
- Gałazkiewicz B., Mossakowska M., Osińska H., Dabrowska R. Polymerization of G-actin by caldesmon. FEBS Lett. 1985 May 6;184(1):144–149. doi: 10.1016/0014-5793(85)80671-2. [DOI] [PubMed] [Google Scholar]
- Gopalakrishna R., Anderson W. B. Ca2+-induced hydrophobic site on calmodulin: application for purification of calmodulin by phenyl-Sepharose affinity chromatography. Biochem Biophys Res Commun. 1982 Jan 29;104(2):830–836. doi: 10.1016/0006-291x(82)90712-4. [DOI] [PubMed] [Google Scholar]
- Ikebe M., Hartshorne D. J. Proteolysis of smooth muscle myosin by Staphylococcus aureus protease: preparation of heavy meromyosin and subfragment 1 with intact 20 000-dalton light chains. Biochemistry. 1985 Apr 23;24(9):2380–2387. doi: 10.1021/bi00330a038. [DOI] [PubMed] [Google Scholar]
- Kakiuchi R., Inui M., Morimoto K., Kanda K., Sobue K., Kakiuchi S. Caldesmon, a calmodulin-binding, F actin-interacting protein, is present in aorta, uterus and platelets. FEBS Lett. 1983 Apr 18;154(2):351–356. doi: 10.1016/0014-5793(83)80181-1. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Marston S. B., Lehman W. Caldesmon is a Ca2+-regulatory component of native smooth-muscle thin filaments. Biochem J. 1985 Nov 1;231(3):517–522. doi: 10.1042/bj2310517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marston S. B., Moody C., Smith C. Mechanisms of the regulation of myofibrillar function in vascular smooth muscle. Biochem Soc Trans. 1984 Dec;12(6):945–948. doi: 10.1042/bst0120945. [DOI] [PubMed] [Google Scholar]
- Marston S. B., Smith C. W. Purification and properties of Ca2+-regulated thin filaments and F-actin from sheep aorta smooth muscle. J Muscle Res Cell Motil. 1984 Oct;5(5):559–575. doi: 10.1007/BF00713261. [DOI] [PubMed] [Google Scholar]
- Moody C. J., Marston S. B., Smith C. W. Bundling of actin filaments by aorta caldesmon is not related to its regulatory function. FEBS Lett. 1985 Oct 21;191(1):107–112. doi: 10.1016/0014-5793(85)81003-6. [DOI] [PubMed] [Google Scholar]
- Ngai P. K., Carruthers C. A., Walsh M. P. Isolation of the native form of chicken gizzard myosin light-chain kinase. Biochem J. 1984 Mar 15;218(3):863–870. doi: 10.1042/bj2180863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ngai P. K., Walsh M. P. Detection of caldesmon in muscle and non-muscle tissues of the chicken using polyclonal antibodies. Biochem Biophys Res Commun. 1985 Mar 15;127(2):533–539. doi: 10.1016/s0006-291x(85)80192-3. [DOI] [PubMed] [Google Scholar]
- Ngai P. K., Walsh M. P. Inhibition of smooth muscle actin-activated myosin Mg2+-ATPase activity by caldesmon. J Biol Chem. 1984 Nov 25;259(22):13656–13659. [PubMed] [Google Scholar]
- Ngai P. K., Walsh M. P. Properties of caldesmon isolated from chicken gizzard. Biochem J. 1985 Sep 15;230(3):695–707. doi: 10.1042/bj2300695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owada M. K., Hakura A., Iida K., Yahara I., Sobue K., Kakiuchi S. Occurrence of caldesmon (a calmodulin-binding protein) in cultured cells: comparison of normal and transformed cells. Proc Natl Acad Sci U S A. 1984 May;81(10):3133–3137. doi: 10.1073/pnas.81.10.3133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Persechini A., Rowe A. J. Modulation of myosin filament conformation by physiological levels of divalent cation. J Mol Biol. 1984 Jan 5;172(1):23–39. doi: 10.1016/0022-2836(84)90412-1. [DOI] [PubMed] [Google Scholar]
- Persechini A., Stull J. T. Phosphorylation kinetics of skeletal muscle myosin and the effect of phosphorylation on actomyosin adenosinetriphosphatase activity. Biochemistry. 1984 Aug 28;23(18):4144–4150. doi: 10.1021/bi00313a021. [DOI] [PubMed] [Google Scholar]
- Potter J. D. Preparation of troponin and its subunits. Methods Enzymol. 1982;85(Pt B):241–263. doi: 10.1016/0076-6879(82)85024-6. [DOI] [PubMed] [Google Scholar]
- Smillie L. B. Preparation and identification of alpha- and beta-tropomyosins. Methods Enzymol. 1982;85(Pt B):234–241. doi: 10.1016/0076-6879(82)85023-4. [DOI] [PubMed] [Google Scholar]
- Sobue K., Muramoto Y., Fujita M., Kakiuchi S. Purification of a calmodulin-binding protein from chicken gizzard that interacts with F-actin. Proc Natl Acad Sci U S A. 1981 Sep;78(9):5652–5655. doi: 10.1073/pnas.78.9.5652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spector T. Refinement of the coomassie blue method of protein quantitation. A simple and linear spectrophotometric assay for less than or equal to 0.5 to 50 microgram of protein. Anal Biochem. 1978 May;86(1):142–146. doi: 10.1016/0003-2697(78)90327-5. [DOI] [PubMed] [Google Scholar]
- Walsh M. P., Hinkins S., Dabrowska R., Hartshorne D. J. Smooth muscle myosin light chain kinase. Methods Enzymol. 1983;99:279–288. doi: 10.1016/0076-6879(83)99063-8. [DOI] [PubMed] [Google Scholar]
- Walsh M. P., Hinkins S., Flink I. L., Hartshorne D. J. Bovine stomach myosin light chain kinase: purification, characterization, and comparison with the turkey gizzard enzyme. Biochemistry. 1982 Dec 21;21(26):6890–6896. doi: 10.1021/bi00269a041. [DOI] [PubMed] [Google Scholar]
- Walsh M. P., Valentine K. A., Ngai P. K., Carruthers C. A., Hollenberg M. D. Ca2+-dependent hydrophobic-interaction chromatography. Isolation of a novel Ca2+-binding protein and protein kinase C from bovine brain. Biochem J. 1984 Nov 15;224(1):117–127. doi: 10.1042/bj2240117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zot H. G., Potter J. D. Purification of actin from cardiac muscle. Prep Biochem. 1981;11(4):381–395. doi: 10.1080/00327488108065530. [DOI] [PubMed] [Google Scholar]