Abstract
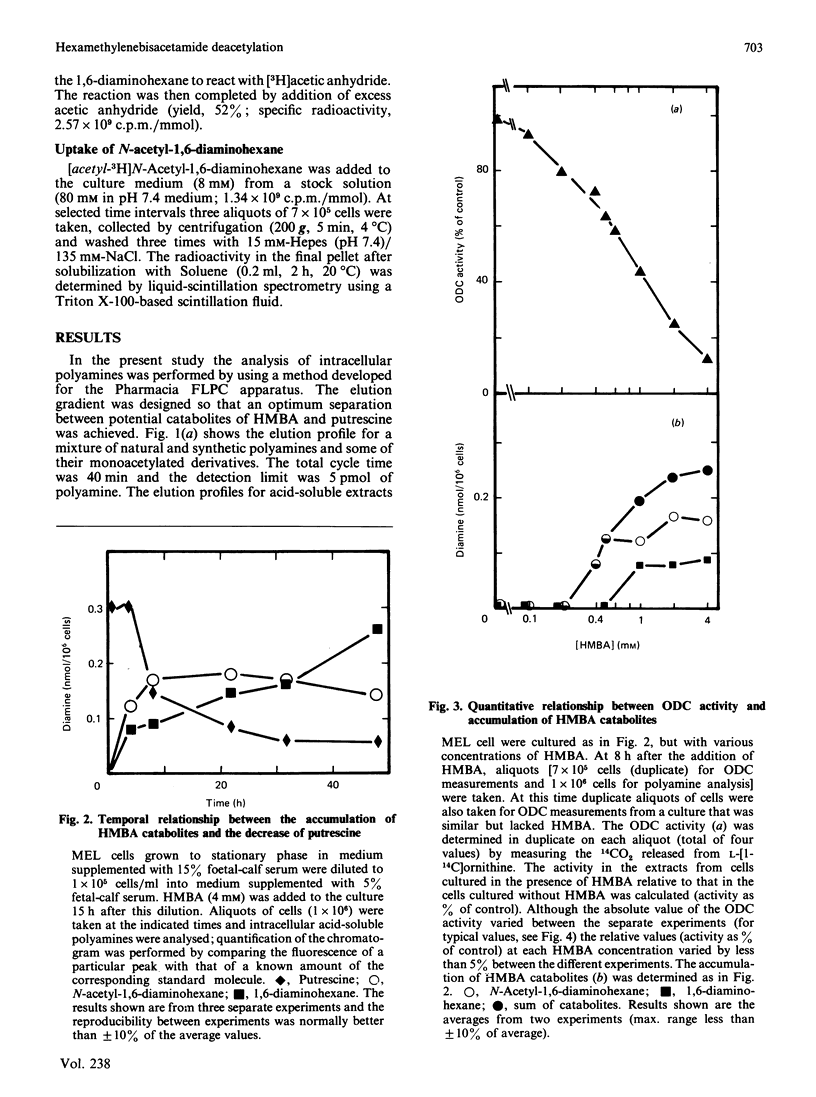
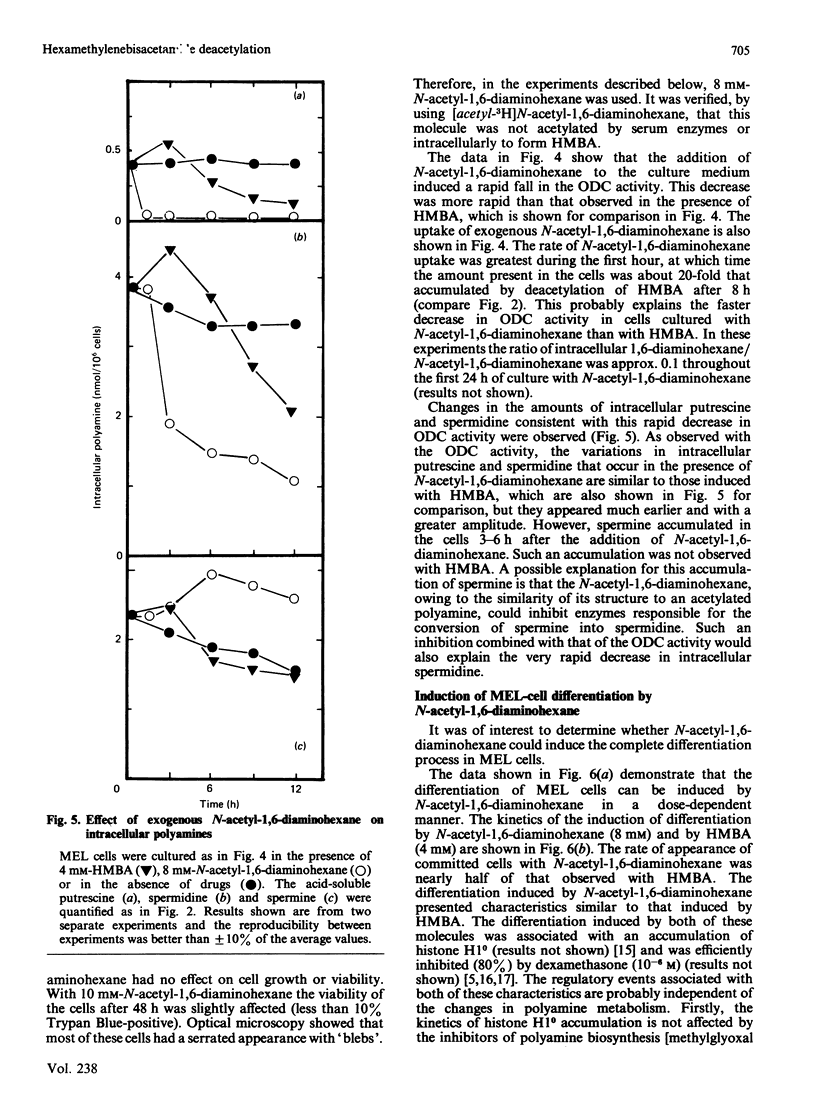
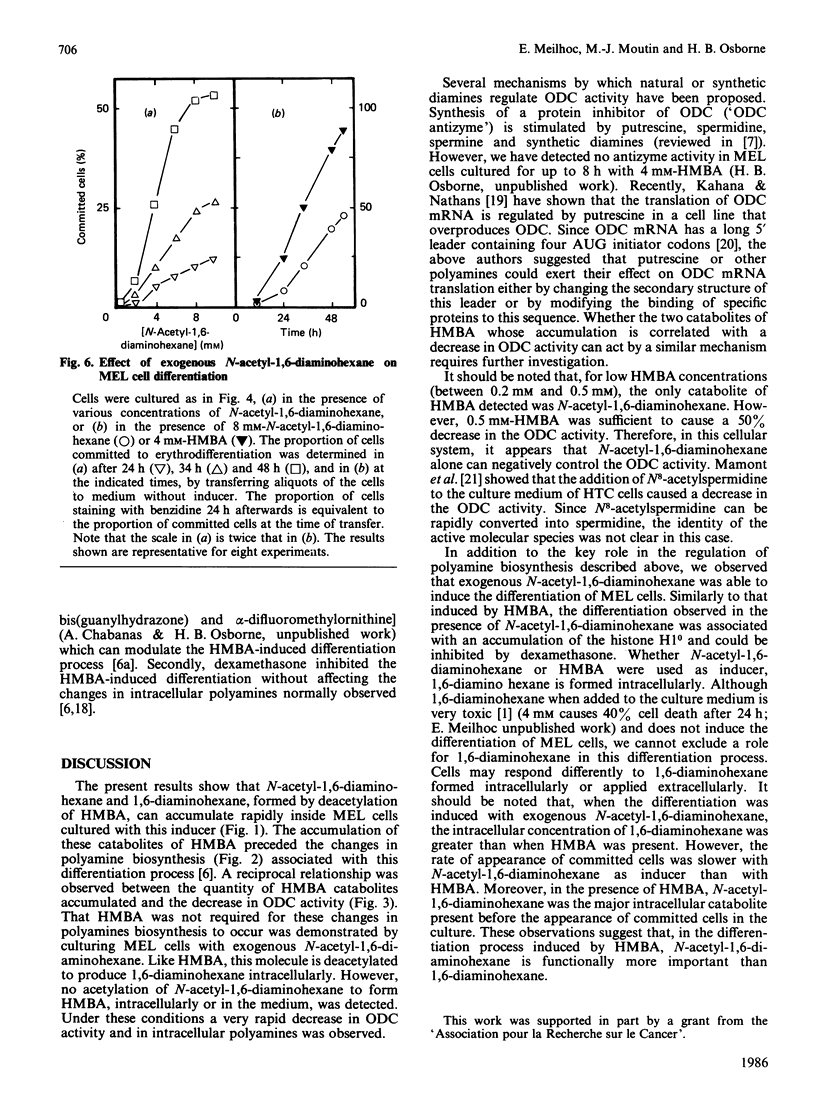
N-Acetyl-1,6-diaminohexane and 1,6-diaminohexane, formed by deacetylation of the inducer hexamethylenebisacetamide (HMBA), are shown to accumulate rapidly inside murine erythroleukaemic cells. The appearance of these molecules preceded the differentiation-associated changes in intracellular polyamines. A quantitative relationship was observed between the accumulation of these molecules and the changes in intracellular polyamines. In the absence of HMBA, exogenous N-acetyl-1,6-diaminohexane was able not only to cause changes in polyamine biosynthesis, but also to induce the complete differentiation process. These results imply that these catabolites of HMBA are directly responsible for the changes in polyamine biosynthesis and probably also for initiating other events regulatory for the differentiation of these cells.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Canellakis Z. N., Marsh L. L., Young P., Bondy P. K. Polyamine metabolism in differentiating Friend erythroleukemia cells. Cancer Res. 1984 Sep;44(9):3841–3845. [PubMed] [Google Scholar]
- Chen Z., Banks J., Rifkind R. A., Marks P. A. Inducer-mediated commitment of murine erythroleukemia cells to differentiation: a multistep process. Proc Natl Acad Sci U S A. 1982 Jan;79(2):471–475. doi: 10.1073/pnas.79.2.471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gazitt Y., Friend C. Polyamine biosynthesis enzymes in the induction and inhibition of differentiation in Friend erythroleukemia cells. Cancer Res. 1980 May;40(5):1727–1732. [PubMed] [Google Scholar]
- Hughes E. H., Schut H. A., Thorgeirsson S. S. Effects of hexamethylene bisacetamide on alpha-fetoprotein, albumin, and transferrin production by two rat hepatoma cell lines. In Vitro. 1982 Feb;18(2):157–164. doi: 10.1007/BF02796408. [DOI] [PubMed] [Google Scholar]
- Hugues B., Osborne H. B. Dexamethasone inhibits a heme-independent event necessary for terminal differentiation of murine erythroleukemia cells. Biochem Biophys Res Commun. 1981 Oct 30;102(4):1342–1349. doi: 10.1016/s0006-291x(81)80159-3. [DOI] [PubMed] [Google Scholar]
- Johns E. W. Studies on histones. 7. Preparative methods for histone fractions from calf thymus. Biochem J. 1964 Jul;92(1):55–59. doi: 10.1042/bj0920055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahana C., Nathans D. Nucleotide sequence of murine ornithine decarboxylase mRNA. Proc Natl Acad Sci U S A. 1985 Mar;82(6):1673–1677. doi: 10.1073/pnas.82.6.1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahana C., Nathans D. Translational regulation of mammalian ornithine decarboxylase by polyamines. J Biol Chem. 1985 Dec 15;260(29):15390–15393. [PubMed] [Google Scholar]
- Mamont P. S., Joder-Ohlenbusch A. M., Siat M. Metabolism of N8-monoacetylspermidine in rat hepatoma cells. Investigation of its effect on the activity of L-ornithine decarboxylase. Eur J Biochem. 1983 Jul 1;133(3):613–616. doi: 10.1111/j.1432-1033.1983.tb07506.x. [DOI] [PubMed] [Google Scholar]
- Meilhoc E., Moutin M. J., Romani B. H., Osborne H. B. Relationship between ornithine decarboxylase activity and hexamethylene bisacetamide-induced differentiation of murine erythroleukemia cells. Exp Cell Res. 1986 Jan;162(1):142–150. doi: 10.1016/0014-4827(86)90432-5. [DOI] [PubMed] [Google Scholar]
- Osborne H. B., Bakke A. C., Yu J. Effect of dexamethasone on hexamethylene bisacetamide-induced Friend cell erythrodifferentiation. Cancer Res. 1982 Feb;42(2):513–518. [PubMed] [Google Scholar]
- Osborne H. B., Chabanas A. Kinetics of histone H10 accumulation and commitment to differentiation in murine erythroleukemia cells. Exp Cell Res. 1984 Jun;152(2):449–458. doi: 10.1016/0014-4827(84)90646-3. [DOI] [PubMed] [Google Scholar]
- Palfrey C., Kimhi Y., Littauer U. Z. Induction of differentiation in mouse neuroblastoma cells by hexamethylene bisacetamide. Biochem Biophys Res Commun. 1977 Jun 6;76(3):937–942. doi: 10.1016/0006-291x(77)91592-3. [DOI] [PubMed] [Google Scholar]
- Paulin D., Perreau J., Jakob H., Jacob F., Yaniv M. Tropomyosin synthesis accompanies formation of actin filaments in embryonal carcinoma cells induced to differentiate by hexamethylene bisacetamide. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1891–1895. doi: 10.1073/pnas.76.4.1891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabson A. S., Stern R., Tralka T. S., Costa J., Wilczek J. Hexamethylene bisacetamide induces morphologic changes and increased synthesis of procollagen in cell line from glioblastoma multiforme. Proc Natl Acad Sci U S A. 1977 Nov;74(11):5060–5064. doi: 10.1073/pnas.74.11.5060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuben R. C. Studies on the mechanism of action of hexamethylene bisacetamide, a potent inducer of erythroleukemic differentiation. Biochim Biophys Acta. 1979 Dec 11;588(3):310–321. doi: 10.1016/0304-4165(79)90339-8. [DOI] [PubMed] [Google Scholar]
- Reuben R. C., Wife R. L., Breslow R., Rifkind R. A., Marks P. A. A new group of potent inducers of differentiation in murine erythroleukemia cells. Proc Natl Acad Sci U S A. 1976 Mar;73(3):862–866. doi: 10.1073/pnas.73.3.862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seiler N., Knödgen B. High-performance liquid chromatographic procedure for the simultaneous determination of the natural polyamines and their monoacetyl derivatives. J Chromatogr. 1980 Dec 12;221(2):227–235. doi: 10.1016/s0378-4347(00)84307-8. [DOI] [PubMed] [Google Scholar]
- Sugiura M., Shafman T., Kufe D. Effects of polyamine depletion on proliferation and differentiation of murine erythroleukemia cells. Cancer Res. 1984 Apr;44(4):1440–1444. [PubMed] [Google Scholar]
- Watanabe T., Shafman T., Kufe D. W. Requirement of spermidine for induction of both heme synthesis and globin transcription in murine erythroleukemia cells. J Cell Physiol. 1985 Mar;122(3):435–440. doi: 10.1002/jcp.1041220314. [DOI] [PubMed] [Google Scholar]


