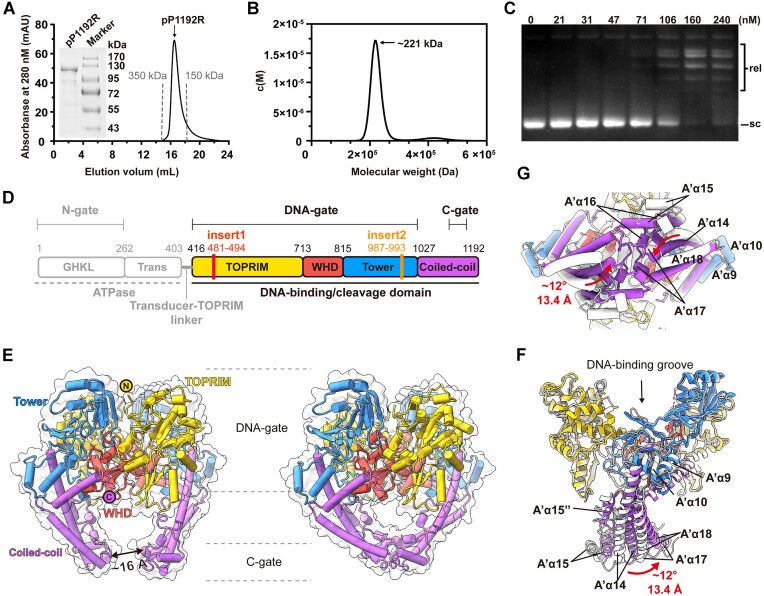
Figure 1.
Biochemical characteristics and overall architectures of ASFV pP1192R in the apo state. (A) The gel filtration profile of pP1192R protein is shown in black, with gray dotted lines marking the elution peaks of two standard proteins. (B) Sedimentation velocity profile of pP1192R. Sedimentation coefficient distributions c(M) calculated from the sedimentation velocity profile with the calculated molecular weight shown. (C) Relaxation assays catalyzed by pP1192R. pP1192R was tested at increasing concentrations as indicated above each lane. Relaxed (rel) and supercoiled (sc) forms of DNA are indicated. (D) The schematic representation of pP1192R. Functional domains, dimerization gates, and sequence insertions are labeled. The domain organization of the DNA binding/cleavage domain is highlighted. (E) The overall structures of dimeric pP1192R DNA binding/cleavage domain, featuring its C-gate in either the open (left) or closed (right) state. Each domain shown in the cartoon representation is colored as in (D) and the surface representation is colored white. (F and G) Conformational changes of the pP1192R C-gate between open (white) and closed (colored as shown in (D)). The secondary structural elements are labelled according to Supplementary Figure 2C.