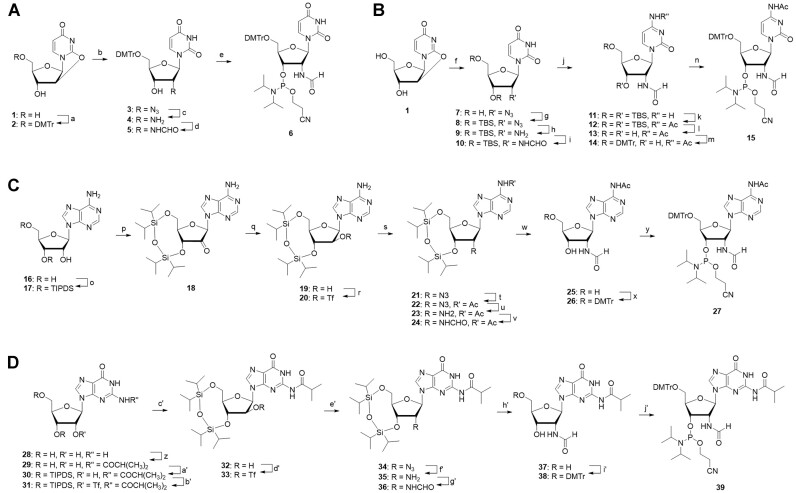
Scheme 1.
Synthesis of 2′-formamidonucleoside phosphoroamidite analogs. Reagents and conditions: (a) DMTrCl, DMAP, pyridine, rt, 90%; (b) NaN3, 15-crown-5, DMF, 120°C, 65%; (c) H2, Pd/C, MeOH, rt, 91%; (d) formic acid, EDC-HCl, DMAP, DIPEA, DCM, rt, 57%; (e) 2-cyanoethyl-N, N-diisopropylchlorophosphoroamidite, DIPEA, DCM, 0°C, 29%; (f) NaN3, 15-crown-5, DMF, 120°C; (g) TBDMSCl, imidazole, DMF, rt, 41% (over 2 steps); (h) H2, Pd/C, MeOH, rt, 99%; (i) formic acid, EDC-HCl, DMAP, DIPEA, DCM, rt, 85%; (j) TPSCl, TEA, DMAP, NH4OH, ACN, 0°C, 87%; (k) acetic anhydride, pyridine, rt; (l) TEA-3HF, THF, rt, 65% (over 2 sateps); (m) DMTrCl, DMAP, pyridine, rt, 95%; (n) 2-cyanoethyl-N,N-diisopropylchlorophosphoroamidite, DIPEA, DCM, 0°C, 76%; (o) TIPDSCl2, pyridine, rt, 99%; (p) CrO3, pyridine, acetic anhydride, DCM, rt; (q) NaBH4, EtOH/H2O, 0°C, 29% (over 2 steps); (r) N-phenylbis (trifluoromethanesulfonimide), DMAP, DCM, 0°C, 97%; (s) NaN3, DMF, 60°C; (t) acetyl chloride, pyridine, rt, 63% (over 2 steps); (u) H2, Pd/C, MeOH, rt, 68%; (v) formic acid, EDC-HCl, DMAP, DIPEA, DCM, rt, 91%; (w) TEA-3HF, THF, rt, 98%; (x) DMTrCl, DMAP, pyridine, rt, 97%; (y) 2-cyanoethyl-N,N-diisopropylchlorophosphoroamidite, DIPEA, DCM, 0°C, 63%; (z) 1) TMSCl, pyridine 2) isobutyryl chloride, 3) NH4OH, rt, 93%; (a’) TIPDSCl2, pyridine, rt, 96%; (b’) CF3SO2Cl, DMAP, DCM, 0°C, 28%; (c’) CF3COOK, DIPEA, DMF, 80°C, 53%, (d’) CF3SO2Cl, DMAP, DCM, 0°C, 20%; (e’) NaN3, DMF, rt, 95%; (f’) H2, Pd/C, MeOH, rt, 53%; (g’) formic acid, EDC-HCl, DMAP, DIPEA, DCM, rt, 96%; (h’) TEA-3HF, THF, rt, 94%; (i’) DMTrCl, DMAP, pyridine, rt, 97%; (j’) 2-cyanoethyl-N,N-diisopropylchlorophosphoroamidite, DIPEA, DCM, 0°C, 50%.