Highlights
-
•
Anal squamous cell cancer patients without distant metastasis who received immunotherapy along with radical concurrent chemoradiotherapy (CRT) experienced significantly higher 3-year PFS, DMFS and LRFS rates than those treated with surgery or CRT alone.
-
•
The addition of immunotherapy to CRT for locally advanced anal squamous cell cancer may further enhance disease-related survival benefits, without adding unmanageable toxicity.
-
•
The role of PD-L1 expression might be a predictive biomarker to inform the clinical use of immunotherapy for anal squamous cell cancer.
Keywords: Surgery, Chemoradiotherapy, Immunotherapy, Squamous cell carcinoma, Anal canal
Abstract
The current standard of care for anal squamous cell carcinoma (ASCC) is definitive concurrent chemoradiotherapy (CRT). However, about a third of patients may experience treatment failure. Recently, immunotherapy has emerged as a novel strategy for metastatic ASCC patients. We evaluated the efficacy and safety of surgery, CRT alone, and CRT with immunotherapy (CRT-I) in 100 nonmetastatic ASCC patients, treated from April 2012 through May 2023, by determining survival outcomes and acute adverse events. The median (range) follow-up was 30.7 (7.6 to 134.9) months. The study cohort 3-year overall survival (OS), progression-free survival (PFS), distant metastasis-free survival (DMFS), and locoregional recurrence-free survival (LRFS) rates were 80.7 %, 62.2 %, 71.1 %, and 67.6 %, respectively. The Surgery group had significantly lower rates than the CRT and CRT-I groups for 3-year PFS (33.1% vs. 65.2% vs. 92.9 %, P < 0.001), DMFS (46.7% vs. 74.6% vs. 92.9 %, P = 0.002) and LRFS (37.0% vs. 73.3% vs. 92.9 %, P < 0.001), respectively. All patients receiving CRT-I were alive at last follow-up. Of 100 patients, 26 (26.0 %) experienced severe (≥ grade 3) acute toxicity. Of 24 patients receiving CRT-I, 8 (33.3 %) had severe acute toxicity. Using immunohistochemistry, peritumoural stromal infiltration by CD8+ T cells was significantly higher after CRT-I compared to before CRT-I and to after CRT alone. The addition of immunotherapy to CRT may be an effective first-line treatment option with favourable survival outcomes and acceptable toxicity for patients with ASCC. A prospective, randomized trial assessing the efficacy of CRT combined with a PD-1 inhibitor in patients with locally advanced ASCC is in progress.
Implications for Practice.
Definitive concurrent chemoradiotherapy (CRT) has been more recommended than surgery for anal squamous cell cancer (ASCC) patients. However, more than one-third of patients may experience treatment failure. In this study 100 ASCC patients without distant metastasis who received immunotherapy along with CRT experienced significantly higher 3-year PFS, DMFS and LRFS rates than those treated with surgery or CRT alone. The addition of immunotherapy to CRT for non-metastatic ASCC has the potential to improve survival.
Alt-text: Unlabelled box
Introduction
Anal cancer is a rare malignancy, but the incidence has been increasing for decades in China, as well as in other developed countries [1,2]. Squamous cell carcinoma accounts for over 80 % of the histological subtypes of anal cancer and is strongly associated with human papillomavirus (HPV) infection [3]. The current standard treatment for nonmetastatic anal squamous cell carcinoma (ASCC) is definitive concurrent chemoradiotherapy (CRT). Although CRT has achieved a higher than 80 % complete response (CR) rate in patients with ASCC, between 25 % and 40 % of patients eventually experience treatment failures [4]. Therefore, there is a need for additional treatment options that might improve outcomes in patients with ASCC.
Patients who are immunocompromised, including those with persistent HPV infection, are at higher risk of developing anal cancer [5]. At the same time, patients with an HPV-positive status have better responses to CRT and to immunotherapy, likely because of their tumours have higher immunogenicity [6]. Moreover, immune checkpoint inhibitors (ICIs) have emerged as a novel strategy for managing patients with previously treated metastatic ASCC, having produced encouraging rates of response and durable survival [[7], [8], [9]]. More recently, a comprehensive treatment strategy for ASCC that includes CRT and immunotherapy has attracted increasing attention, as this might offer potential advantages over treatment with CRT alone. The addition of immunotherapy in the form of a Programmed cell death-1 (PD-1)/programmed death-ligand 1 (PD-L1) checkpoint inhibitor might induce an increased immunogenic effect by initiating cell death, phagocytosis, and tumour antigen release, all of which might lead to the reactivation of immune-mediated tumour surveillance and enhanced anti-tumour activity [10,11]. Indeed, prospective, clinical trials to assess the efficacy of ICIs combined with CRT for ASCC are currently in progress (e.g., NCT03233711, NCT04230759, NCT05661188, and NCT05374252).
To date, there have been only a few publications reporting on the use of CRT combined with immunotherapy, and these have only provided preliminary evidence of the safety of concurrent administration of CRT and immunotherapy in patients with cancers of the lung and the anus [[12], [13], [14]]. The first aim of our study was to evaluate both the efficacy and safety of surgery, CRT alone, and CRT with immunotherapy in patients with ASCC, by reporting on survival outcomes, treatment responses, and acute treatment-related adverse events. The second aim our study was to use these results to determine whether enough preliminary evidence for the benefits of concurrent CRT with immunotherapy exists to warrant a prospective investigation of the use of this combination for locally advanced ASCC patients.
Materials and methods
Patients
Those enrolled in this study were consecutive patients who were treated between April 2012 and May 2023 at the Sixth Affiliated Hospital of Sun Yat-sen University for newly diagnosed, histologically proven, nonmetastatic ASCC.
All patients underwent anorectal palpation, enhanced pelvic MRI, and enhanced CT of the chest and abdomen as part of their initial diagnosis. All women underwent gynaecologic examination to exclude primary gynaecologic tumours. Patients were included in the study who (a) had biopsy-proven ASCC, and (b) had no distant metastases. Patients were excluded from the study who (a) had a second primary tumour, (b) refused CRT and/or curative surgery (i.e., chose palliative therapy), (c) had metastatic or recurrent ASCC. Clinical stages were assigned according to the TNM staging system from the American Joint Cancer Committee (AJCC) Cancer Staging Manual, 8th Edition. This study was approved by the Ethics Committee of the Sun Yat-sen University Cancer Centre (No. 2023ZSLYEC-667).
Treatments
The patients in the study were divided into 3 groups based on the primary treatment received according to attending physician recommendations: Surgery, CRT, and CRT plus immunotherapy (CRT-I) (Fig. 1).
Fig. 1.
The CONSORT diagram. Abbreviations: ASCC, anal squamous cell carcinoma; CRT, chemoradiotherapy; CRT-I, chemoradiotherapy plus immunotherapy; CR, complete response; PR, partial response; AC, adjuvant chemotherapy; W & W, watch and wait; PCR, pathological complete response.
Surgery
For patients with clinical stage T1 disease, local excision was performed. For patients with advanced localized disease (i.e., clinical stage T2-T4 or N-positive), Miles’ (abdominoperineal resection) surgery was performed. In addition, for patients with persistent disease after CRT or with recurrent disease during follow-up, local excision, or Miles’ surgery were performed, with the choice of the type of surgery being based on the stage and location of the tumour.
Radiotherapy
Patients receiving definitive CRT all had intensity-modulated radiotherapy (IMRT), and they were treated 5 days a week with 1 fraction per day. Patients with early disease (i.e., clinical stage T1 or T2) received the following IMRT regimen: a total dose of 50 to 54 Gy to the planning target volume (PTV) of the gross tumour volume of the primary tumour (GTVp), given in 25 to 27 fractions at 2.0 Gy per fraction; and, 45 Gy to the PTV of the clinical target volume (CTV), given in 25 fractions at 1.8 Gy per fraction. Patients with advanced disease (i.e., clinical stage T3-T4 or N1) received the following IMRT regimen: an initial dose of 45 Gy to the PTV of the clinical target volume (CTV) given in 25 fractions at 1.8 Gy per fraction; a new sim-CT following the end of the 45 Gy course of radiotherapy; and, a boost dose of 10 to 14 Gy given in 5 to 10 fractions at 2.0 Gy per fraction to the volume of the shrunken GTVp and metastatic lymph nodes (for a total dose of 54 to 59 Gy).
The CTV was defined as the GTV plus areas considered at significant risk of harbouring microscopic disease, including a 1-cm margin around the GTV and anal canal. This included the mesorectum (perirectal fascia), perirectal nodes, presacral region, internal iliac lymph nodes, external iliac lymph nodes, and inguinal lymph nodes. In addition, other adjacent organs were included when invaded by primary tumour.
Chemotherapy
Chemotherapy consisted of concurrent (during radiotherapy) and/or adjuvant (after radiotherapy or surgery) regimens. The regimen chosen for each patient was determined by the attending physician and multi-disciplinary cancer team at our hospital. Patients receiving CRT or CRT-I all received 2 cycles of concurrent chemotherapy, either on days 1 and 22 or on days 1 and 29.
Of the 100 patients in the study, 72 (72.0 %) received between 1 and 4 cycles of adjuvant chemotherapy. Among the 24 patients receiving Surgery, 12 (50.0 %) patients received adjuvant chemotherapy, ranging from 1 to 4 cycles. Among the 76 patients receiving CRT or CRT-I, 60 (78.9 %) received adjuvant chemotherapy, ranging from 1 to 2 cycles.
Concurrent and adjuvant chemotherapy regimens that were used included: 5-fluorouracil (5-FU) with mitomycin C (MMC); 5-FU with cisplatin; or paclitaxel with cisplatin. The doses used were based on recommendations from the National Comprehensive Cancer Network (NCCN), Clinical Practice Guidelines in Oncology for ASCC.
Immunotherapy
The PD-1 antibody (sintilimab, 200 mg/dose intravenously) was used for immunotherapy. All patients in the CRT-I group received 2 single-dose cycles of concurrent (during radiotherapy) immunotherapy, given on days 1 and 22. All of these patients also received adjuvant immunotherapy given every 3 weeks, ranging from 1 to 2 cycles, based on the recommendation of their attending physician. Thus, patients who had immunotherapy in the study received a total of 3 to 4 cycles.
Tumour microenvironments
Tumour or tumour-bed biopsies of 21 randomly chosen study patients in the Surgery, CRT, and CRT-I groups (Supplementary Table 1) were evaluated with immunohistochemistry (IHC) to assess the tumour microenvironments (TME) of these patients. The intratumoural tissue prior to treatment was used to study the relationship between the expression of PD-L1 and the infiltration of immunocytes (i.e., CD3+, CD4+, and CD8+ T cells, as well as CD163+ M2 macrophages).
The tissue from the patients in the CRT and CRT-I groups was used to discuss the infiltration of immunocytes in the intratumoural region and peritumoural stroma. Six of the patients from the CRT-I group had no residual tumour after treatment. Therefore, for the tissue of the CRT-I group after treatment, we opted to do these comparisons using the infiltration of immunocytes in the peritumoural stroma.
The specific biomarkers used for IHC included CD3 (Rabbit, ZSJQ#ZA-0503), CD4 (Rabbit, MXB#RMA-0620), CD8 (Rabbit, MXB#Kit-0026), CD163 (Rabbit, Abcam#ab182422), and PD-L1 (Rabbit, Abcam#ab213524). The relative expression of CD3, CD4, CD8, and CD163 was determined by randomly choosing 3 views of intratumour and peritumoural stromal regions at a 20x magnification and then estimating the area of positively stained immunocytes as a percentage of the total area of intratumour regions or peritumoural stroma. For PD-L1, the TAP (Tumour Area Positivity) Score was determined by randomly choosing 3 views of intratumour regions at a 20x magnification and estimating the area of positively stained tumour cells and immunocytes as a percentage of the total tumour (tumour and stromal cells) area. PD-L1 positivity was defined as a relative expression of PD-L1 in intratumour regions of 1 % or higher.
Follow-up
During the course of CRT, patients were evaluated weekly, including for any toxicity. Laboratory tests including complete blood count with differential, platelet count, and both renal and liver function tests were performed before each cycle of concurrent chemotherapy and immunotherapy. Adverse events and acute toxicities were monitored for at least 3 months after the completion of radiotherapy or surgery, graded using the Common Terminology Criteria for Adverse Events version 4.0 (CTCAE v4.0), and recorded in the medical record. To report toxicity outcomes, the maximum toxic effect grade was used for each patient and each event type.
The duration of follow-up was defined as the time from the first day of any treatment to either the date of the last follow-up examination or the date of death. The initial assessment of tumour response was performed using clinical examination, enhanced pelvic MRI, and transrectal ultrasonography 3 months after surgery or the end of radiotherapy. A similar assessment was performed 6 months after surgery or the end of the radiotherapy, with the purpose of determining clinical efficacy. A clinical complete response (cCR) was defined as the absence of primary or nodal tumour by digital examination, colonoscopy, and enhanced MRI of the pelvis. If there was evidence of persistent disease, this was confirmed by biopsy, and then salvage local excision or surgery was performed. Subsequently, patients had repeat assessments at 3-month intervals during the first 2 years, and at 6-month intervals thereafter.
Outcomes
Primary outcomes determined in this study were overall survival (OS), progression-free survival (PFS), distant metastasis-free survival (DMFS), and locoregional relapse-free survival (LRFS). OS was defined as the interval from the initiation of treatment to death as a result of any cause; PFS was defined as the interval from the initiation of treatment to locoregional recurrence or metastasis, or death. DMFS was defined as the interval from the initiation of treatment to the first distant relapse or death; and, LRFS was defined as the interval from the initiation of treatment to the first locoregional relapse or death. Secondary outcomes determined were treatment response, and acute treatment-related toxicity.
Statistical methods
Either the chi-squared (χ2) test or Fisher's exact test was used to compare the distributions of the patient demographic and clinicopathological characteristics between the 2 groups. Kaplan-Meier survival curves were used to compare patient survival outcomes between the 2 groups. Statistical differences between curves were calculated using the log-rank test. Linear regression was used for analysis of correlation between the intratumoural infiltration of immunocytes and expression of PD-L1 prior to treatment. The Mann-Whitney test was used to compare the infiltration of immunocytes and the expression of PD-L1 in the intratumoural and peritumoural stromal regions before and after treatment. All survival outcome measures were censored on September 1, 2023. All P values were two-sided, and a P value <0.05 was considered statistically significant. Statistical analyses were performed with SPSS (version 24.0; SPSS, Inc., Chicago, IL).
Results
Patient characteristics
A total of 100 patients with ASCC were included in this study (Fig. 1). The median age of all patients was 55 (range, 22 to 86) years (Table 1). Of the 100 patients, 80 (80.0 %) were women. As well, 10 (10 %) patients had stage I, 46 (46 %) had stage II, and 44 (44 %) had stage III disease. Of 83 patients evaluated for HPV, 68 (81.9 %) patients were HPV-positive. Patients were divided into 3 groups based on their initial treatment modality, as follows: 24 (24.0 %) patients were in the Surgery group, 52 (52 %) patients were in the CRT group, and 24 (24.0 %) patients were in the CRT-I group.
Table 1.
Demographic and clinicopathological characteristics of 100 patients with anal squamous cell carcinoma, by primary treatment modality, April 2012 through May 2023.
| Characteristics |
Total patients (N = 100) |
Surgery group (N = 24) |
CRT group (N = 52) |
CRT-I group (N = 24) |
P |
|---|---|---|---|---|---|
| n (%) | n (%) | n (%) | n (%) | ||
| Age, years (median, 55) | 0.624 | ||||
| ≤ 55 | 54 (54.0) | 12 (50.0) | 27 (51.9) | 15 (62.5) | |
| > 55 | 46 (46.0) | 12 (50.0) | 25 (48.1) | 9 (37.5) | |
| Gender | 0.760 | ||||
| Male | 20 (20.0) | 6 (25.0) | 10 (19.2) | 4 (16.7) | |
| Female | 80 (80.0) | 18 (75.0) | 42 (80.8) | 20 (83.3) | |
| HPV status | 0.561 | ||||
| Positive | 68 (68.0) | 14 (58.3) | 35 (67.3) | 19 (79.2) | |
| Negative | 15 (15.0) | 4 (16.7) | 9 (17.3) | 2 (8.3) | |
| NT | 17 (17.0) | 6 (25.0) | 8 (15.4) | 3 (12.5) | |
| Tumor differentiation (initial biopsy) | 0.275 | ||||
| High | 11 (11.0) | 3 (12.5) | 8 (15.4) | 1 (4.2) | |
| Moderate | 58 (58.0) | 17 (70.8) | 26 (50.0) | 15 (58.3) | |
| Poor | 31 (31.0) | 4 (16.7) | 18 (34.6) | 9 (37.5) | |
| Clinical T stage | 0.001 | ||||
| cT1 | 15 (15.0) | 8 (33.3) | 5 (9.6) | 2 (8.3) | |
| cT2 | 41 (41.0) | 2 (8.4) | 27 (51.9) | 12 (50.0) | |
| cT3 | 24 (24.0) | 9 (37.5) | 13 (25.0) | 2 (8.4) | |
| cT4 | 20 (20.0) | 5 (20.8) | 7 (13.5) | 8 (33.3) | |
| Clinical N stage | 0.059 | ||||
| cN0 | 25 (25.0) | 10 (41.7) | 12 (23.1) | 3 (12.5) | |
| cN1 | 75 (75.0) | 14 (58.3) | 40 (76.9) | 21 (87.5) | |
| Clinical TNM stage | <0.001 | ||||
| I | 10 (10.0) | 7 (29.2) | 3 (5.8) | 0 (0.0) | |
| II | 46 (46.0) | 3 (12.5) | 29 (55.7) | 14 (58.3) | |
| III | 44 (44.0) | 14 (58.3) | 20 (38.5) | 10 (41.7) | |
| Chemotherapy regimena | <0.001 | ||||
| MMC + 5-FU | 36 (36.0) | 1 (4.2) | 21 (40.3) | 14 (58.3) | |
| Cisplatin + 5-FU | 21 (21.0) | 7 (29.1) | 11 (21.2) | 3 (12.5) | |
| Paclitaxel + cisplatin | 31 (31.0) | 4 (16.7) | 20 (38.5) | 7 (29.2) | |
| None | 12 (12.0) | 12 (50.0) | 0 (0.0) | 0 (0.0) | |
| Total chemotherapy cycles, median (IQR) | 3 (2–4) | 2 (0–4) | 3 (2–4) | 3 (3–4) | — |
Includes both concurrent and adjuvant chemotherapy; patients continued with the same regimen throughout the study.
Abbreviations: CRT, chemoradiotherapy; CRT-I, chemoradiotherapy with immunotherapy; NT, not tested; MMC, mitomycin C; 5-FU, 5-fluorouracil; IQR, interquartile range.
Patient clinicopathological characteristics were compared among the 3 treatment modality groups (Table 1). Compared with those in the other 2 groups, a significantly higher proportion of patients in the CRT-I group had advanced clinical T stages (i.e., cT4) (P = 0.001) as well as advanced clinical stages (i.e., stage II and III) (P < 0.001).
Short-term (6-month) treatment efficacy
At the assessment 6 months after completing primary treatment, of the 100 patients in the study, 74 (74.0 %) had retained their anus while 26 (26.0 %) had received a colostomy, either as part of the primary Miles’ surgery (n = 17) or as part of Miles’ surgery for persistent disease (n = 9) (Fig. 1). The rates of colostomy for patients in the Surgery, CRT, and CRT-I groups were 70.8 %, 17.3 %, and 0 %, respectively. Of the 76 patients who received CRT or CRT-I, a total of 51 (67.1 %) achieved a cCR. Meanwhile, of the 12 patients in the CRT group who received salvage local excision, 3 achieved a pCR. Thus, a total of 54 (71.1 %) of the 76 patients achieved a CR. In addition, 9 patients required a colostomy as part of Miles’ Surgery for residual tumour.
Treatment toxicity and tolerance
Of the 100 patients in the study, 25 (25.0 %) patients required a break during treatment because of acute toxicity. All 76 patients who started radiotherapy completed it. In the study cohort, the most common grade 1 or 2 acute toxicity was radiation dermatitis occurring in 67 patients, followed by haematological toxicity in 42 patients, and gastrointestinal toxicity in 30 patients (Table 2).
Table 2.
Acute toxicities of treatments in 100 patients with anal squamous cell carcinoma, by primary treatment modality, April 2012 through May 2023.
| Acute adverse effects |
Surgery group (N = 24) |
CRT group (N = 52) |
CRT-I group (N = 24) |
P |
|---|---|---|---|---|
| n (%) | n (%) | n (%) | ||
| Haematological toxicity | 0.522 | |||
| Grade 0 | 9 (37.4) | 15 (28.8) | 7 (29.2) | |
| Grade 1–2 | 14 (58.4) | 30 (57.7) | 12 (50.0) | |
| Grade 3–4 | 1 (4.2) | 7 (13.5) | 5 (20.8) | |
| Gastrointestinal toxicity | 0.433 | |||
| Grade 0 | 14 (58.3) | 24 (46.2) | 14 (58.3) | |
| Grade 1–2 | 10 (41.7) | 22 (42.3) | 8 (33.4) | |
| Grade 3–4 | 0 (0.0) | 6 (11.5) | 2 (8.3) | |
| Radiation dermatitis | <0.001 | |||
| Grade 0 | 24 (100.0) | 3 (5.8) | 2 (8.3) | |
| Grade 1–2 | 0 (0.0) | 46 (88.5) | 21 (87.5) | |
| Grade 3–4 | 0 (0.0) | 3 (5.7) | 1 (4.2) | |
| Peripheral neurotoxicity | 0.285 | |||
| Grade 0 | 21 (87.5) | 34 (65.4) | 19 (79.2) | |
| Grade 1–2 | 3 (12.5) | 17 (32.7) | 5 (20.8) | |
| Grade 3–4 | 0 (0.0) | 1 (1.9) | 0 (0.0) | |
| Immune-related toxicity | 0.054 | |||
| Grade 0 | 24 (100.0) | 52 (100.0) | 22 (91.7) | |
| Grade 1–2 | 0 (0.0) | 0 (0.0) | 2 (8.3) | |
| Grade 3–4 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
Abbreviations: CRT, chemoradiotherapy; CRT-I, chemoradiotherapy with immunotherapy.
Overall, 26 (26.0 %) patients experienced grade 3 or 4 acute toxicity (Table 2). Of the 24 patients in the Surgery group, only 1 (4.2 %) had a high-grade toxicity (i.e., grade 3 haematological toxicity). Of the 52 patients in the CRT group, 17 (32.3 %) experienced grade 3 or 4 toxicity. Of the 24 patients in the CRT-I group, 7 (29.2 %) had grade 3 haematological or gastrointestinal toxicity, and 1 (4.2 %) had grade 4 radiation dermatitis. Taken together, grade 3 or 4 toxicities were experienced by 32.3 % and 33.3 % of the patients in the CRT and CRT-I groups, respectively. While the most common immune-related toxicity in patients in the CRT-I group was hypothyroidism in 3 patients, it was only grade 2 and resolved after a short course of corticosteroids. Furthermore, no grade 3 or 4 immune-related toxicity occurred in patients in the CRT-I group.
Survival outcomes
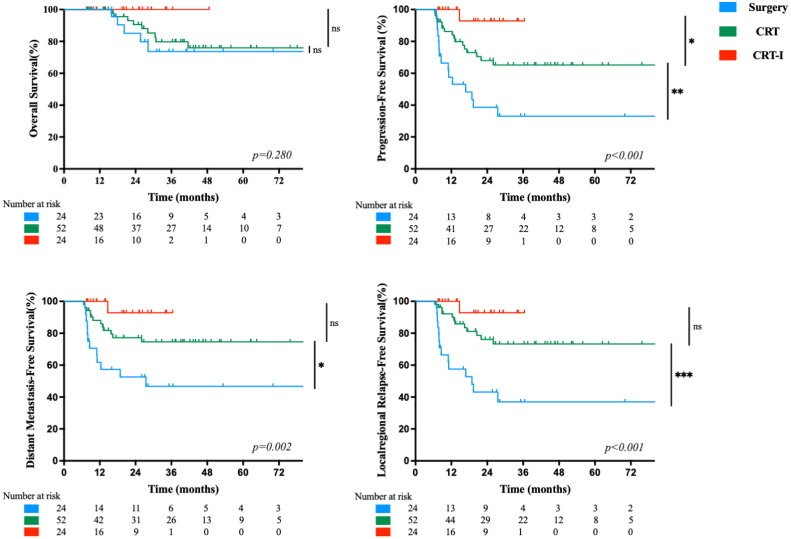
The median (range) follow-up for the study cohort was 30.7 (7.6 to 134.9) months. The estimated 3-year OS, PFS, DMFS, and LRFS rates for all patients were 80.7 %, 62.2 %, 71.1 %, and 67.6 %, respectively (Fig. 2). The Surgery group had significantly lower rates than the CRT and CRT-I groups for 3-year PFS (33.1% vs. 65.2% vs. 92.9 %, P < 0.001), DMFS (46.7% vs. 74.6% vs. 92.9 %, P = 0.002) and LRFS (37.0% vs. 73.3% vs. 92.9 %, P < 0.001), respectively. The Surgery group also had a lower 3-year OS rate (73.7 %) than the CRT (79.8 %) and CRT-I (100 %) groups, but these differences were not statistically significant (P = 0.280) (Fig. 3).
Fig. 2.
Kaplan-Meier curve analyses of 100 patients with anal squamous cell carcinoma, who received for primary treatment either surgery, concurrent chemoradiotherapy (CRT), or concurrent chemoradiotherapy plus immunotherapy (CRT-I), from April 2012 through May 2023. Curves show rates of (A) overall survival, (B) progression-free survival, (C) distant metastasis-free survival, and (D) locoregional relapse-free survival.
Fig. 3.
Kaplan-Meier curve analyses of patients with anal squamous cell carcinoma, treated between April 2012 and May 2023, showing survival after surgery, concurrent chemoradiotherapy (CRT), or CRT plus immunotherapy (CRT-I). Curves show comparisons of rates, by type of treatment, for all 100 study patients, of (A) overall survival (P = 0.280), (B) progression-free survival (P < 0.001), (C) distant metastasis-free survival (P = 0.002), and (D) locoregional relapse-free survival (P < 0.001). Symbols represent treatment comparisons between two groups: Numbers of symbols indicate P-value levels: ns indicates not significant, ‘*’ means P < 0.05, ‘**’ means P < 0.01 and ‘***’ means P < 0.001.
Tumour microenvironments
Before treatment, the intratumoural expression of PD-L1 was significantly positively correlated with the intratumoural infiltration of CD3+ T cells, CD4+ T cells, and CD8+ T cells, but it was not significantly correlated with the intratumoural infiltration of CD163+ M2 macrophages (Fig. 4A). The positive rate of intratumoural expression of PD-L1 in the CRT and CRT-I groups was 50.0 % and 33.3 %, respectively, but these did not differ significantly (Fig. 4B-C).
Fig. 4.
Analysis of the tumor microenvironments (TME) using immunohistochemistry (IHC) in 21 randomly selected patients with anal squamous cell carcinoma whose primary treatment was either surgery (n = 4), chemoradiotherapy (CRT) (n = 9), or chemoradiotherapy, before and after treatment. (A-C) IHC analyses of intratumoural tissue of 21 patients with anal squamous cell carcinoma. (A) Correlation analysis between intratumoural expression of PD-L1 and infiltration of different immunocytes of patients before treatment. (B) Bar graph of comparison of intratumoural expression of PD-L1 between patients in the CRT and CRT-I groups before treatment (P = 0.414). (C) Representative IHC staining images of intratumoural expression of PD-L1 before treatment of (left) CRT patient 98 and (right) CRT-I patient 99. (D-G) IHC analyses of tissue of the 9 patients treated with CRT and the 8 patients treated with CRT-Ion was evaluated, comparing it between 2 treatment groups, and within each treatment group, both before and after treatment. (D) Bar graphs of comparison between CRT and CRT-I groups of peritumoural stromal infiltration of CD8+ T cells (left) before treatment (P = 0.573) and (right) after treatment (P = 0.031). (E) Representative IHC staining images of infiltration of CD8+ T cells in peritumoral stroma before treatment of (left) CRT patient 98 and (right) CRT-I patient 84. (F) Bar graphs of comparison before and after treatment of infiltration of CD8+ T cells in the peritumoral stroma within the (left) CRT group (P = 0.963) and (right) CRT-I group (P = 0.008). (G) Representative IHC staining images of infiltration of CD8+ T cells in peritumoral stroma after treatment of (left) CRT patient 98 and (right) CRT-I patient 84. Correlation analysis done using linear regression. Comparisons done using Mann-Whitney Test. In Bar graphs, bars represent medians (50th percentile), horizontal lines represent interquartile ranges (75th and 25th percentiles), ns indicates not significant, ‘*’ is P < 0.05 and ‘**’ is P < 0.01.
In comparing the CRT and CRT-I groups, there was no significant difference between the 2 groups in the pre-treatment infiltration of immunocytes, including CD8+ T cells, in both the peritumoural stromal (Fig. 4D) and intratumoural (Supplementary Figure 1) regions. However, the post-treatment CD8+ T cell infiltration in the peritumoural stroma in the CRT-I group was significantly higher than that in the CRT group (Fig. 4D, Fig. 4, Fig. 4).
In comparing pre- and post-treatment in the CRT group, there was no significant difference in the peritumoural stromal (Fig. 4E-G) or intratumoural (Supplementary Figure 1) infiltration of CD 8 + T cells. Conversely, in the CRT-I group, the infiltration of CD8+ T cells in the peritumoural stroma after treatment was significantly higher than before treatment (Fig. 4E-G).
As for the other immunocytes (i.e., CD3+ T cells, CD4+ T cells, and CD163+ M2 macrophages), when comparing both their peritumoural stromal and intratumoural infiltration pre- and post-treatment within the CRT and CRT-I treatment groups, there were no significant differences (Supplementary Figures 2 and 1A-D). Also, when comparing the 2 treatment groups with each other before treatment, there were no significant differences in the peritumoural or intratumoural infiltration of any of these cells (Supplementary Figures 2 and 1E-H).
Discussion
In this retrospective analysis, we compared the efficacy and safety of surgery, CRT, and CRT-I for patients who had ASCC, most of whom had locally advanced disease. Patients in our study whose primary treatment was either CRT or CRT-I had significantly higher 3-year PFS, DMFS and LRFS rates than those primarily treated with surgery. In particular, the 3-year PFS, DMFS and LRFS rates in patients who received immunotherapy with CRT were both 92.9 %, while in those who received CRT alone were 65.2 %, 74.6 % and 73.3 %, respectively. In addition, the patients receiving CRT-I did not have substantially worse treatment-related toxicity than those receiving CRT alone. Finally, the short-term colostomy-free rates for patients who had CRT-I, CRT, and surgery were 100 %, 82.7 %, and 29.2 %, respectively.
Survival rates were the primary outcomes used in our study. Xiao WW, et al. were the first to study survival and safety of the neoadjuvant use of PD-1 antibody prior to CRT in 5 patients with ASCC [15]. Yuan, et al. reported on 59 patients with ASCC in south China, whose 3-year OS and PFS rates were 74.2 % and 73.4 %, respectively [2]. In addition, in 940 patients with anal cancer who were involved in the ACT II study and divided into 2 groups, the 3-year PFS rates were 73 % and 74 % [15]. The 3-year OS, PFS, DMFS, and LRFS rates for our study cohort were 80.7 %, 62.2 %, 71.1 %, and 67.6 %, respectively. These survival outcomes are comparable to previous studies. More importantly, we observed particularly promising survival outcomes in our patients who received CRT with immunotherapy. With our median follow-up of <36 months, the OS results of our study were immature. However, all patients receiving CRT-I in our study were alive at last follow-up. Furthermore, the estimated 3-year DMFS and LRFS rates for those receiving CRT-I in our study were both 92.9 %, while the rates for CRT alone were 74.6 % and 73.3 %, respectively. Although these preliminary results do not provide definitive evidence for the superiority of CRT-I, our group will be carrying out a prospective, randomized trial to determine this, by comparing the efficacy of CRT to CRT combined with a concurrent and adjuvant PD-1 inhibitor in patients with locally advanced ASCC (NCT 05,374,252).
In our study, 67.1 % of the patients treated with CRT or CRT-I had a cCR. These results are inferior to those obtained by others. Emma, et al. reported that 92.5 % of their patients with ASCC who were treated with definitive CRT achieved a cCR [16]. In their study, 35.4 % of the patients presented with advanced stage disease and about 50 % had N1 disease. In contrast, 44 % patients of our patients had an advanced T stage (T3 or T4) and 75.0 % had lymph node metastases. The latter are associated with poor survival in patients with ASCC [17]. These characteristics of the patients in our study likely contributed to the less favourable short-term efficacy results in our analysis.
The safety profile reported for patients in our study receiving CRT or CRT-I was consistent with that observed in other investigations involving CRT. Furthermore, the patients in our study receiving CRT-I had acute toxicity rates that were similar to those of the patients receiving CRT without immunotherapy. In the RTOG 0529 study, patients with locally advanced ASCC were treated with CRT, including dose-painted IMRT, and 73 % had acute grade 2+ haematologic toxicity, 21 % had grade 3+ gastrointestinal toxicity, and 23 % had grade 3+ dermatologic toxicity, suggesting significant sparing of these toxicities relative to when conventional radiotherapy was used as part of CRT [18]. Conversely in our study, of the patients receiving CRT or CRT-I, which included dose-painted IMRT, grade 3+ haematologic toxicity occurred in 15.8 %, grade 3+ gastrointestinal toxicity occurred in 10.5 %, and grade 3+ dermatologic toxicity occurred in 5.2 %. Finally, all of the adverse event results taken together suggest that the addition of immunotherapy to CRT did not substantially increase what were manageable toxicities for CRT alone.
We further analysed the tumour microenvironment in our patients, because it has been shown to play a significant role in the effectiveness of immunotherapy for certain cancers. Wakeham, et al. demonstrated that positivity for p16, a surrogate for HPV, and higher levels of tumour-infiltrating lymphocytes (TILs) in the tumours of patients with anal cancer were associated with improved survival [19]. Another study of patients with ASCC treated with CRT showed that those whose tumours at baseline had higher amounts of infiltration by a particular TIL, the CD8+ T cell, had significantly better rates of local control, DFS, and OS [6]. In our study, we observed that the amount of CD8+ T cell infiltration in the peritumoural stroma was significantly increased after CRT-I treatment relative to before treatment. We also observed that while there was no significant difference between the CRT and CRT-I groups in the amount of pre-treatment CD8+ T cell infiltration in the peritumoural stroma, the amount of post-treatment CD8+ T cell infiltration was significantly higher in the CRT-I group than in the CRT group. Although the implications of these results are limited by the small sample size, they do suggest that the addition of immunotherapy to CRT may significantly activate tumour immunity, and it is possible that such activation may correlate with more favourable survival outcomes.
The expression of PD-L1 within tumours has been reported to be a predictive biomarker for the response to immunotherapy in other advanced or metastatic squamous cell cancers, including cervical[20] and oesophageal[21] cancers. For ASCC, relative research has yet to be conducted. In the Keynote-158 trial using pembrolizumab to treat ASCC, the group of patients with PD-L1-positive tumours had a better overall response rate (ORR) than the group with PD-L1-negative tumours (15% vs. 3 %, respectively) [7]. However, in a study involving 37 patients with treatment-refractory ASCC who received nivolumab, patients were not selected based on PD-L1 expression, and yet the ORR rate for the cohort was 24 % [8]. In our study, we observed that the expression of PD-L1 was positively correlated with infiltration by TILs, including CD3+, CD4+, and CD8+ T cells. These results suggest that the role of PD-L1 expression might be a predictive biomarker to inform the clinical use of immunotherapy for ASCC.
Limitations
Our work had some limitations. First, the retrospective design of this analysis and the heterogeneity among physician practices at our institution might have resulted in selection bias. Second, although the study was focused on CRT with or without immunotherapy, we did include patients who underwent surgery as primary treatment. We decided to include these patients because, like those receiving CRT or CRT-I, they had the same diagnosis and were treated with curative intent. As expected and kept in mind during the analysis of results, the patients who had surgery as primary treatment had significantly worse disease control than those who received CRT or CRT-I. Finally, the median follow-up in the study was relatively short at just <36 months. However, the ACT II trial reported a relapse rate of <1 % after 3 years [22], suggesting that short-term efficacy may reasonably predict long-term survival.
Conclusions
For patients with locally advanced ASCC, CRT appears to provide disease-related survival benefits over surgery. For those same patients, the addition of immunotherapy to CRT may result in further disease-related survival benefits, without adding unmanageable toxicity. These preliminary findings have led to our design of a prospective, randomized trial comparing the efficacy of CRT to CRT combined with a concurrent and adjuvant PD-1 inhibitor in patients with locally advanced ASCC.
Ethics approval
The experimental protocol was approved by the Central Ethics Committee of the Sixth Affiliated Hospital, Sun Yat-sen University (No. 2023ZSLYEC-667).
Registry and the registration no of the study/trial
N/A.
Animal studies
N/A.
Consent to participate
N/A.
Consent for publication
Not applicable.
Funding
This work was supported by the National Natural Science Foundation of China (No. 81972885) and the program of Guangdong Provincial Clinical Research Centre for Digestive Diseases (2020B1111170004).
CRediT authorship contribution statement
Fang He: Writing – review & editing, Writing – original draft. Mo Chen: Writing – original draft, Methodology, Conceptualization. Qi-jun Yao: Supervision, Methodology, Conceptualization. Zhi-min Liu: Supervision, Resources. Yandong Zhao: Validation, Supervision. Fengyun Pei: Validation, Supervision. Jian Zheng: Data curation, Supervision. Yuan-hong Gao: Resources, Project administration, Data curation, Conceptualization. Jun Huang: Writing – review & editing, Supervision, Funding acquisition.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Availability of data and material
The datasets used for this study are available from the corresponding author upon reasonable request.
Acknowledgements
N/A.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.tranon.2024.102133.
Contributor Information
Yuan-hong Gao, Email: gaoyh@mail.sysu.edu.cn.
Jun Huang, Email: huangj97@mail.sysu.edu.cn.
Appendix. Supplementary materials
References
- 1.Siegel R.L., Miller K.D., Wagle N.S., Jemal A. Cancer statistics, 2023. CA Cancer J. Clin. 2023;73:17–48. doi: 10.3322/caac.21763. [DOI] [PubMed] [Google Scholar]
- 2.Yuan Y., Xie W.H., Li R.Z., et al. Comprehensive treatment experience of anal squamous cell carcinoma from a tertiary cancer center in South China. Cancer Med. 2022;11:117–127. doi: 10.1002/cam4.4433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lin C., Franceschi S., Clifford G.M. Human papillomavirus types from infection to cancer in the anus, according to sex and HIV status: a systematic review and meta-analysis. Lancet Infect. Dis. 2018;18:198–206. doi: 10.1016/S1473-3099(17)30653-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Eng C., Ciombor K.K., Cho M., et al. Anal Cancer: emerging Standards in a Rare Disease. J. Clin. Oncol. 2022;40:2774–2788. doi: 10.1200/JCO.21.02566. [DOI] [PubMed] [Google Scholar]
- 5.Daling J.R., Madeleine M.M., Johnson L.G., et al. Human papillomavirus, smoking, and sexual practices in the etiology of anal cancer. Cancer. 2004;101:270–280. doi: 10.1002/cncr.20365. [DOI] [PubMed] [Google Scholar]
- 6.Balermpas P., Martin D., Wieland U., et al. Human papilloma virus load and PD-1/PD-L1, CD8(+) and FOXP3 in anal cancer patients treated with chemoradiotherapy: rationale for immunotherapy. Oncoimmunology. 2017;6 doi: 10.1080/2162402X.2017.1288331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Marabelle A., Cassier P.A., Fakih M., et al. Pembrolizumab for previously treated advanced anal squamous cell carcinoma: results from the non-randomised, multicohort, multicentre, phase 2 KEYNOTE-158 study. Lancet Gastroenterol. Hepatol. 2022;7:446–454. doi: 10.1016/S2468-1253(21)00382-4. [DOI] [PubMed] [Google Scholar]
- 8.Morris V.K., Salem M.E., Nimeiri H., et al. Nivolumab for previously treated unresectable metastatic anal cancer (NCI9673): a multicentre, single-arm, phase 2 study. Lancet Oncol. 2017;18:446–453. doi: 10.1016/S1470-2045(17)30104-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim S., Ghiringhelli F., de la Fouchardière C., et al. Atezolizumab plus modified docetaxel, cisplatin, and fluorouracil as first-line treatment for advanced anal cancer (SCARCE C17-02 PRODIGE 60): a randomised, non-comparative, phase 2 study. Lancet Oncol. 2024;25:518–528. doi: 10.1016/S1470-2045(24)00081-0. [DOI] [PubMed] [Google Scholar]
- 10.Sharabi A.B., Lim M., DeWeese T.L., Drake C.G. Radiation and checkpoint blockade immunotherapy: radiosensitisation and potential mechanisms of synergy. Lancet Oncol. 2015;16:e498–e509. doi: 10.1016/S1470-2045(15)00007-8. [DOI] [PubMed] [Google Scholar]
- 11.Pauken K.E., Wherry E.J. Overcoming T cell exhaustion in infection and cancer. Trends. Immunol. 2015;36:265–276. doi: 10.1016/j.it.2015.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rizvi N.A., Hellmann M.D., Brahmer J.R., et al. Nivolumab in combination with platinum-based doublet chemotherapy for first-line treatment of advanced non–small-cell lung cancer. J. Clin. Oncol. 2016;34:2969–2979. doi: 10.1200/JCO.2016.66.9861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jabbour S.K., Berman A.T., Decker R.H., et al. Prospective phase I multi-institutional trial of PD-1 blockade with pembrolizumab during concurrent chemoradiation for locally advanced, unresectable non-small cell lung cancer. J. Clin. Oncol. 2019;37:8511. [Google Scholar]
- 14.Xiao W., Yuan Y., Wang S., et al. Neoadjuvant PD-1 blockade combined with chemotherapy followed by concurrent immunoradiotherapy in locally advanced anal canal squamous cell carcinoma patients: antitumor efficacy. Safety .Biomarker Analysis. Front. Immunol. 2021;12 doi: 10.3389/fimmu.2021.798451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.James R.D., Glynne-Jones R., Meadows H.M., et al. Mitomycin or cisplatin chemoradiation with or without maintenance chemotherapy for treatment of squamous-cell carcinoma of the anus (ACT II): a randomised, phase 3, open-label, 2 × 2 factorial trial. Lancet Oncol. 2013;14:516–524. doi: 10.1016/S1470-2045(13)70086-X. [DOI] [PubMed] [Google Scholar]
- 16.Holliday E.B., Morris V.K., Johnson B., et al. Definitive intensity-modulated chemoradiation for anal squamous cell carcinoma: outcomes and toxicity of 428 patients treated at a single institution. Oncologist. 2022;27:40–47. doi: 10.1093/oncolo/oyab006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Theophanous S., Samuel R., Lilley J., et al. Prognostic factors for patients with anal cancer treated with conformal radiotherapy-a systematic review. BMC. Cancer. 2022;22:607. doi: 10.1186/s12885-022-09729-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kachnic L.A., Winter K., Myerson R.J., et al. RTOG 0529: a phase 2 evaluation of dose-painted intensity modulated radiation therapy in combination with 5-fluorouracil and mitomycin-C for the reduction of acute morbidity in carcinoma of the anal canal. Int. J. Radiat. Oncol. Biol. Phys. 2013;86:27–33. doi: 10.1016/j.ijrobp.2012.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wakeham K., Murray L., Muirhead R., et al. Multicentre investigation of prognostic factors incorporating p16 and tumour infiltrating lymphocytes for anal cancer after chemoradiotherapy. Clin. Oncol. (R. Coll. Radiol) 2021;33:638–649. doi: 10.1016/j.clon.2021.04.015. [DOI] [PubMed] [Google Scholar]
- 20.Colombo N., Dubot C., Lorusso D., et al. Pembrolizumab for persistent, recurrent, or metastatic cervical cancer. N. Engl. J. Med. 2021;385:1856–1867. doi: 10.1056/NEJMoa2112435. [DOI] [PubMed] [Google Scholar]
- 21.Sun J.M., Shen L., Shah M.A., et al. Pembrolizumab plus chemotherapy versus chemotherapy alone for first-line treatment of advanced oesophageal cancer (KEYNOTE-590): a randomised, placebo-controlled, phase 3 study. Lancet. 2021;398:759–771. doi: 10.1016/S0140-6736(21)01234-4. [DOI] [PubMed] [Google Scholar]
- 22.Glynne-Jones R., Sebag-Montefiore D., Meadows H.M., et al. Best time to assess complete clinical response after chemoradiotherapy in squamous cell carcinoma of the anus (ACT II): a post-hoc analysis of randomised controlled phase 3 trial. Lancet Oncol. 2017;18:347–356. doi: 10.1016/S1470-2045(17)30071-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used for this study are available from the corresponding author upon reasonable request.