Extended Data Fig. 3: Conditions and reproducibility of the Ribo-seq experiments to elucidate the mode of action of EVN.
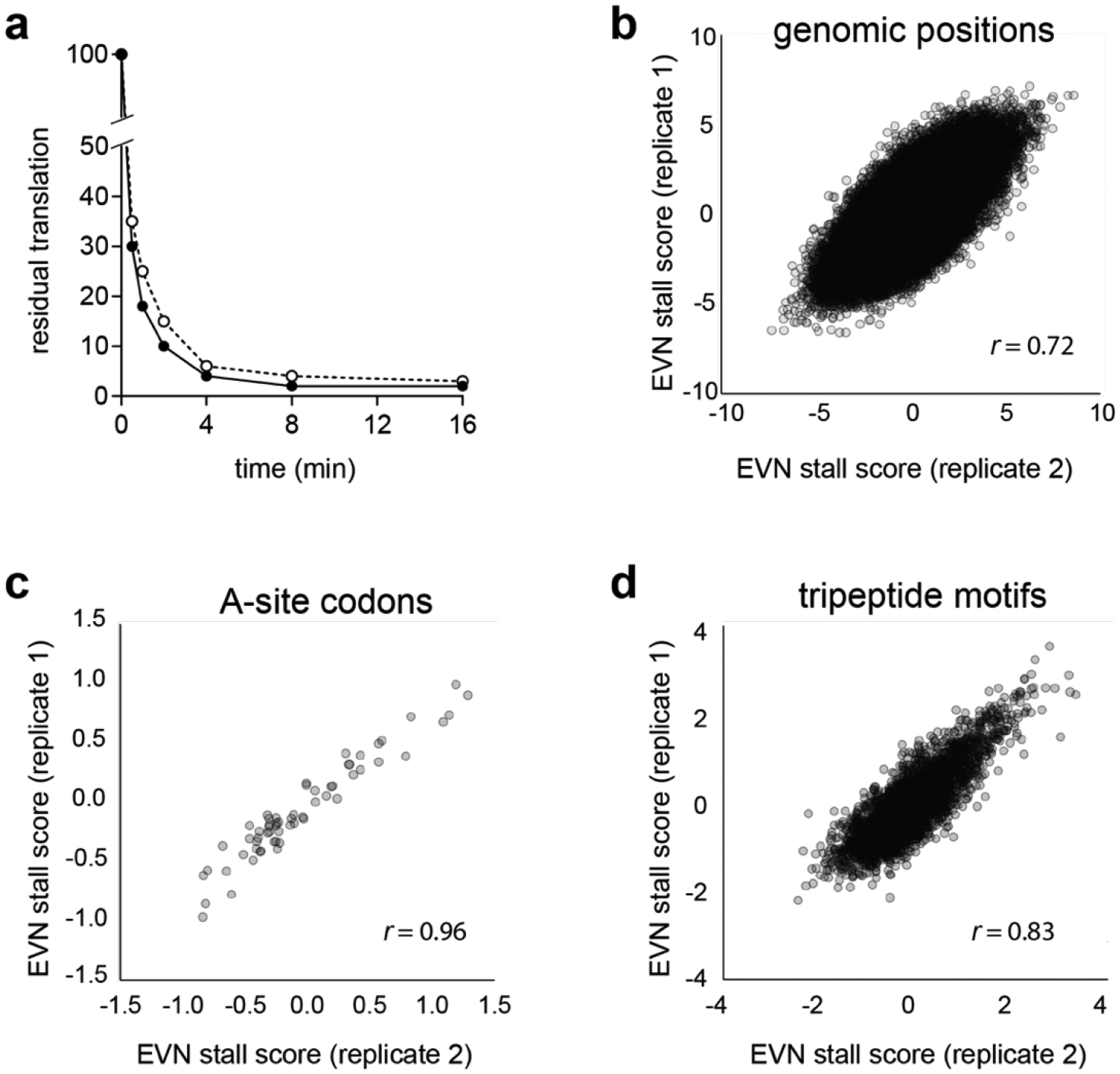
a, Residual global protein synthesis (evaluated as incorporation of [35S]-Met into the TCA-insoluble protein fraction) of E. coli cells exposed to 50 μg/mL EVN for 4 min. Incorporation of [35S]-Met in a sample of the bacterial culture taken right before the addition of EVN was set as 100%. Open and closed circles represent the results of two independent experiments. b-d, Reproducibility of the two independent Ribo-seq experiments as judged by: (b) the EVN stall scores of all the considered codon positions (n = 118,222, Pearson’s r = 0.72); (c) of the mean stall score for codons at the ribosomal A site (n = 61, Pearson’s r = 0.96); (d) of the mean score for considered tripeptide motifs (n = 4039, Pearson’s r = 0.83) described in Figs. 2 and 3.
