Abstract
Elexacaftor/tezacaftor/ivacaftor (ELX/TEZ/IVA) was given US Food and Drug Administration approval based on its therapeutic benefits to treat patients with cystic fibrosis (CF) who had at least 1 allele of the CF transmembrane conductance regulator (CFTR) with phenylalanine deleted at position 508 (F508del). The increase in genotyping studies has increased the frequency of use of CFTR modulators; however, severe allergic reactions to CFTR modulators have also been described. It is critical to avoid the offending medication and select alternative treatments while dealing with drug allergies. Drug desensitization may be taken into consideration in situations where there is no other option. This article describes home desensitization treatment for a patient with CF who developed a maculopapular rash following CFTR modulator medication. There are currently no alternative drugs for CFTR modulators, which are crucial for patients with CF, and limited experience is available with allergic reactions to these drugs. It is important to establish desensitization protocols in order to control drug reactions to CFTR modulators, which are vital for individuals with CF.
Keywords: CFTR modulator, cystic fibrosis, desensitization, ELX/TEZ/IVA, Trikafta
Introduction
Cystic fibrosis (CF) is an autosomal recessive disease caused by mutations in the gene encoding the cystic fibrosis transmembrane regulatory protein, leading to a chloride channel defect that causes multiorgan involvement, including the lung. The aim of treatment is to minimize organ damage and prevent infection 1. CF transmembrane conductance regulator (CFTR) modulators offer therapeutic improvement by restoring the function of defective channels. Studies have demonstrated that in patients with CF who had at least 1 allele of the CFTR with phenylalanine deleted at position 508 (F508del), a combination of the CFTR modulators elexacaftor/tezacaftor/ivacaftor (ELX/TEZ/IVA), known by the trade name Trikafta (Vertex Pharmaceuticals, Boston, MA), dramatically decreased the incidence of pulmonary exacerbations while also improving pulmonary function tests2,3. Nevertheless, rash was seen in 4% to 10% of patients, resulting in 1% to 2% discontinuation4–6. When allergies associated to ELX/TEZ/IVA emerge, there is typically no recognized standard treatment, and management approaches vary widely. Allergic reactions brought on by CFTR modulators might be T-cell–mediated delayed reactions. T-cell clones were obtained from a patient with CF who had an adverse reaction after starting lumacaftor/ivacaftor, and it was revealed that only the clones obtained after lumacaftor were sensitive to the drug, while those taken after ivacaftor and tezacaftor were not7. Additionally, it was noted that these T-cell clones did not exhibit cross-reactivity between lumacaftor and tezacaftor. According to the report, MHC (Major Histocompatibility Complex) class II molecules directly connect to lumacaftor-responsive CD4+ T-cell clones, activating them.
In the development of allergies to essential medicines in cases of chronic usage, desensitization protocols such as the use of a fraction of tablets or compounded aliquots gain importance. In this report, we describe a home desensitization procedure for a patient with CF who had a maculopapular rash after using a CFTR modulator.
Search Strategy
The search was performed through PubMed/MEDLINE, using the following keywords: cystic fibrosis, CFTR modulators, ELX/TEZ/IVA, Trikafta, Elexacaftor, Tezacaftor, Ivacaftor, allergic reaction, hypersensitivity, drug reaction, and drug desensitization, filtered for articles published. The systematic review was performed according to the PRISMA checklist8. We included all articles related to patients with CF who experienced an allergic reaction to CFTR modulators. The titles, abstracts, and entire contents of the relevant articles were examined separately by 2 researchers.
We obtained informed consent from the patient and his family. It was approved by the ethics board (KAEK-157) at the University of Akdeniz, Turkey.
Case
A 16-year-old male patient with widespread maculopapular rash and intense body itching presented to our clinic. Apart from the rash, no mucosal wounds, angioedema, or any systemic involvement were seen. Because the patient was using pancrelipase, budesonide/formoterol, vitamins, and dornase alfa for years, the rash was attributed to an allergic reaction related to ELX/TEZ/IVA that started 10 days prior due to the delta F508/G85E mutation. CFTR modulators were not previously prescribed to the patient.
Two tablets containing VX-445 (elexacaftor–100 mg)/VX-661 (tezacaftor–50 mg)/VX-770 (ivacaftor–75 mg) were prescribed for the patient in the morning, and 1 tablet of ivacaftor (150 mg) in the evening. It was decided to discontinue the ELX/TEZ/IVA treatment owing to the rash. Improvement was shown 4 days after starting therapy with lansoprazole, methylprednisolone, and desloratadine at doses of 30 mg/day, 1 mg/kg/day, and 5 mg/day, respectively. The patient’s viral markers all tested normal. No patch test or skin prick test was carried out. Six weeks after the improvement of symptoms, similar complaints developed after re-exposure to the drug, which was thought to be a drug eruption (Figures 1 and 2). Naranjo Adverse Drug Reaction Probability Scale score of 9 for the patient indicated the presence of a drug-related rash9. It was decided to continue the ELX/TEZ/IVA treatment after drug desensitization because the expected reductions in pulmonary exacerbations with ELX/TEZ/IVA had great importance for our patient. The desensitization protocol is shown in Table 1. The yellow tablet was crushed, and the resulting suspension was diluted with 100 mL of water to create the required liquid at a concentration of 1 mg/mL. We then took 1 mL of this liquid and diluted it once again with 100 mL of water. The prepared 10 mcg/1 mL solution was used in the desensitization process; because of stability problems, the preparation was remade every 24 hours. This desensitization protocol began with the premedication of desloratadine, and the premedication was stopped after the full dose was reached. No reaction was observed.
Figure 1.
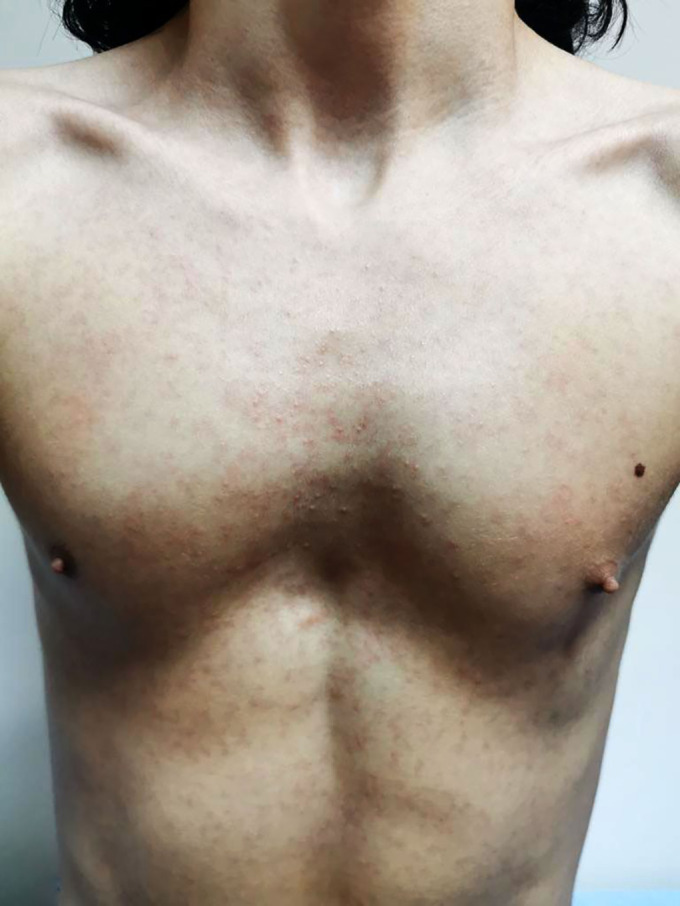
Maculopapular rash.
Figure 2.
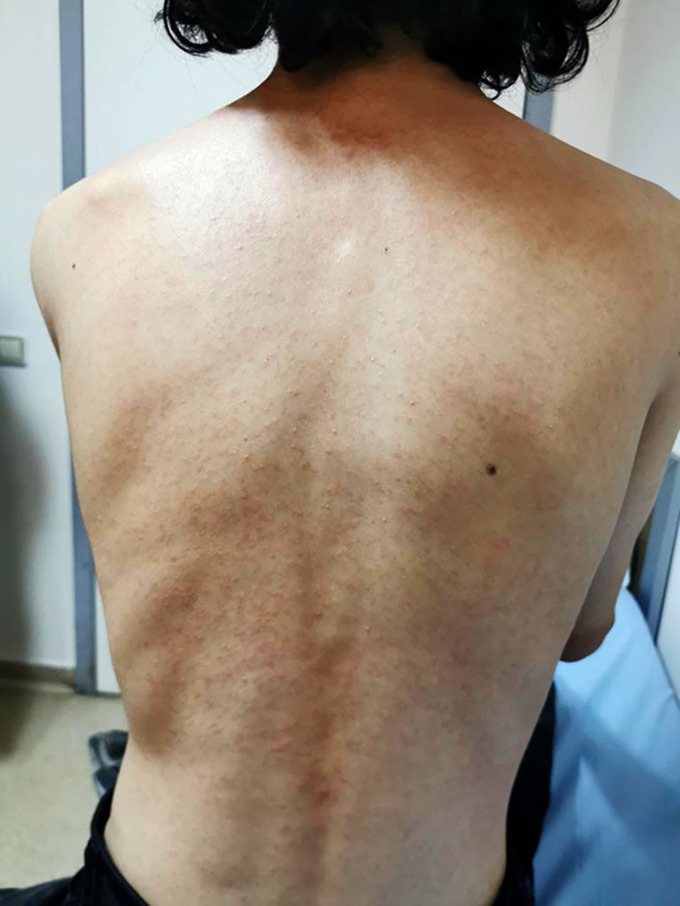
Maculopapular rash.
Table 1.
Trikafta Desensitization Protocol (Adapted From Muirhead15)
| Day(s) | Observation Place and Duration | Dose* | Concentration, mg/mL | Cumulative dose, mg/day | Reaction |
|---|---|---|---|---|---|
| Premedication drug: desloratadine 5 mg/day | |||||
| 1 | Pediatric Allergy Clinic (each dose was increased at 30-min intervals) (yellow tablet) |
10 mcg | 0.2 | 0.42 | None |
| 20 mcg | None | ||||
| 40 mcg | None | ||||
| 100 mcg | None | ||||
| 250 mcg | None | ||||
| 2–7 | Home (yellow tablet) | 500 mcg | 1 | 0.5 | None |
| 8 | Home (each dose was increased at 60-min intervals) (yellow tablet) | 1 mg, 2 mg, 4 mg, 8 mg, 12 mg | 1 | 27 | None |
| 9–14 | Home (yellow tablet) | 25 mg | 1 | 25 | None |
| 15 | Home (each dose was increased at60-min intervals) (yellow tablet) | 30 mg, 35 mg, 35 mg | 1 | 100 | None |
| 16–21 | Home (yellow tablet) | 100 mg (1 yellow tablet) | N/A | 100 | None |
| 22 | Home (each dose was increased at 60-min intervals) (yellow tablet) | 100 mg, 100 mg |
N/A | 200 | None |
| 23 | Home (yellow and blue tablets) | 200 mg (2 yellow tablets) and 1 blue tablet) | N/A | 200 | None |
N/A: not applicable
* Based on dose of elexacaftor. One yellow tablet contains 100-mg elexacaftor/50-mg tezacaftor/75-mg ivacaftor. One blue tablet contains 150-mg ivacaftor.
Discussion
The currently approved CFTR modulators act via 2 different mechanisms: potentiators (ivacaftor) and correctors (lumacaftor, tezacaftor, and elexacaftor). These medications are used in various combinations. Skin rash was observed in 4% to 10% of patients receiving ELX/TEZ/IVA and fewer than 5% of those taking ivacaftor, lumacaftor/ivacaftor, and tezacaftor/ivacaftor in phase 3 clinical trials 4–6. Discontinuation of the drug has been reported to be sufficient in cases with mild drug reactions, while steroid support has also been required in more severe cases. Stashower et al10 reported the case of a 24-year-old female patient who developed erythema multiforme 2 weeks after starting ELX/TEZ/IVA, and Loyd et al.11 described a widespread erythematous macula in a 7-year-old child on the third day, both of whom had clinical improvement upon discontinuation of the drug. However, a 39-year-old male patient described by Mederos-Luis et al.2 required critical care follow-up and needed 20 days of high-dose systemic steroids after developing toxic epidermal necrolysis on the 10th day of therapy. Brennan et al.12 documented the case of a child who required systemic steroid treatment after developing symptoms resembling serum sickness. Adult patients were effectively treated by using desensitization methods, as reported in the studies by Leonhardt et al.13 Patterson et al.,14 and Muirhead et al.15 (Table 2).
Table 2.
Clinical Features, CFTR Modulator Reactions, and Treatment Strategies of Patients With Cystic Fibrosis
| First Author (Reference number) | Country | Number of Patients | Age, yr | Mutation | Sex | Reaction Time After Drug Intake | Symptom | Treatment |
|---|---|---|---|---|---|---|---|---|
| Stashower10 | USA | 1 | 24 | Not specified | F | 2 wk | Pruritic rash | Stop medication |
| Leonhardt13 | USA | 2 | 20, 47 | Phe508del/Phe508del, Phe508del/3849 + 10kbC-T |
2F | 7 days/9 days | Maculopapular rash/red pruritic spot | For case 1 stop medication, methylprednisolone, topical triamcinolone, desensitization; for case 2 stop medication, prednisone, loratadine, desensitization |
| Muirhead15 | USA | 1 | 49 | Homozygous F508del | F | 5 wk | Erythematous, pruritic papules and diffuse edema | Desensitization at home |
| Loyd I11 | USA | 1 | 7 | Not specified | M | 3 days | Diffuse erythematous macules | Stop medication, systemic steroid, acetaminophen and diphenhydramine |
| Mederos-Luis2 | Spain | 1 | 39 | F508del/G542X | M | 10 days | Toxic epidermal necrolysis TEN | Stop medication High-dose systemic steroid |
| Brennan12 | USA | 1 | 12 | Phe508del/Arg347Pro | M | 5 days | Serum sickness–like reaction | Stop medication Systemic steroid |
CFTR, cystic fibrosis transmembrane conductance regulator; TEN, toxic epidermal necrolysis
Approaches in desensitization protocols should be chosen according to the concentration of the drug, the dose range, and the route of administration (oral, intravenous, intramuscular, or subcutaneous). Immunologic tolerance can be maintained with continuous administration of the drug. During the desensitization process, mild reactions or severe reactions ranging up to anaphylaxis may occur. Because the protocol of Muirhead and colleagues15 was designed to be adopted more slowly, it provided the basis for our desensitization protocol. The protocol used by Loyd et al.11 started with half of the required dose and continued with the full dose on the third day. In the protocol of Leonhardt et al.13, the first dose was started at home. However, in that of Muirhead et al.15 which we have also used as a reference, the first dose is given in the hospital environment and continued by increasing the dose in 3 weeks, which makes it safer. The desensitization protocol was successfully applied to our patient, and we were able to continue the ELX/TEZ/IVA treatment. Unlike that of Muirhead and colleagues,15 the preparation of the drug consisted only of dilution with distilled water to a certain concentration.
Because patients with CF lack alternatives in their treatment, drug desensitization may be required in such cases owing to allergic reactions to CFTR modulators. For our patient, we showed that it is important to establish desensitization protocols for drug reactions to CFTR modulators, which are vital for individuals with CF.
ABBREVIATIONS
- CF
cystic fibrosis;
- CFTR
cystic fibrosis transmembrane conductance regulator;
- ELX/TEZ/IVA
elexacaftor/tezacaftor/ivacaftor
Footnotes
Disclosure. The authors declare that there are no conflict of interests. No funding. The authors had full access to all patient information in this report and take responsibility for the integrity and accuracy of the report.
Ethical Approval and Informed Consent. The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national guidelines on human investigation. The authors obtained informed consent from the patient and his family. It was approved by the ethics board (KAEK-157) at the University of Akdeniz, Turkey.
References
- 1.Shteinberg M, Haq IJ, Polineni D, et al. Cystic fibrosis. Lancet . 2021;397(10290):2195–2211. doi: 10.1016/S0140-6736(20)32542-3. [DOI] [PubMed] [Google Scholar]
- 2.Mederos-Luis E, Gonzalez-Perez R, Poza-Guedes P, et al. Toxic epidermal necrolysis induced by cystic fibrosis transmembrane conductance regulator modulators. Contact Dermatitis . 2022;86(3):224–225. doi: 10.1111/cod.14002. [DOI] [PubMed] [Google Scholar]
- 3.Veit G, Roldan A, Hancock MA, et al. Allosteric folding correction of F508del and rare CFTR mutants by elexacaftor-tezacaftor-ivacaftor (Trikafta) combination. JCI Insight . 2020;5(18):e139983. doi: 10.1172/jci.insight.139983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Griese M, Costa S, Linnemann RW, et al. Safety and Efficacy of Elexacaftor/Tezacaftor/Ivacaftor for 24 Weeks or Longer in People with Cystic Fibrosis and One or More F508del Alleles: Interim Results of an Open-Label Phase 3 Clinical Trial. Am J Respir Crit Care Med . 2021;203(3):381–385. doi: 10.1164/rccm.202008-3176LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Heijerman HGM, McKone EF, Downey DG, et al. Efficacy and safety of the elexacaftor plus tezacaftor plus ivacaftor combination regimen in people with cystic fibrosis homozygous for the F508del mutation: a double-blind, randomised, phase 3 trial. Lancet . 2019;394(10212):1940–1948. doi: 10.1016/S0140-6736(19)32597-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zemanick ET, Taylor-Cousar JL, Davies J, et al. A Phase 3 Open-Label Study of Elexacaftor/Tezacaftor/Ivacaftor in Children 6 through 11 Years of Age with Cystic Fibrosis and at Least One F508del Allele. Am J Respir Crit Care Med . 2021;203(12):1522–1532. doi: 10.1164/rccm.202102-0509OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Roehmel JF, Ogese MO, Rohrbach A, et al. Drug allergy to CFTR modulator therapy associated with lumacaftor-specific CD4(+) T lymphocytes. J Allergy Clin Immunol . 2021;147(2):753–756. doi: 10.1016/j.jaci.2020.05.041. [DOI] [PubMed] [Google Scholar]
- 8.Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Syst Rev . 2021;10(1):89. doi: 10.1186/s13643-021-01626-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Naranjo CA, Busto U, Sellers EM, et al. A method for estimating the probability of adverse drug reactions. Clin Pharmacol Ther . 1981;30(2):239–245. doi: 10.1038/clpt.1981.154. [DOI] [PubMed] [Google Scholar]
- 10.Stashower J, Carr P, Miller V, et al. Novel reaction to new cystic fibrosis medication Trikafta. Clin Case Rep . 2021;9(5):e04116. doi: 10.1002/ccr3.4116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Loyd I, Papac N, Hirshburg J, et al. If At First You Don't Succeed, Trikafta Again. J Pediatr Pharmacol Ther . 2022;27(5):467–469. doi: 10.5863/1551-6776-27.5.467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brennan S, Marmor I, Schafer C, et al. Serum sickness-like reaction following initiation of elexacaftor/tezacaftor/ivacaftor therapy. Pediatr Pulmonol . 2020;55(11):2846–2847. doi: 10.1002/ppul.25072. [DOI] [PubMed] [Google Scholar]
- 13.Leonhardt K, Autry EB, Kuhn RJ, et al. CFTR modulator drug desensitization: Preserving the hope of long term improvement. Pediatr Pulmonol . 2021;56(8):2546–2552. doi: 10.1002/ppul.25437. [DOI] [PubMed] [Google Scholar]
- 14.Patterson A, Autry E, Kuhn R, et al. Ivacaftor drug desensitization. Pediatr Pulmonol . 2019;54(6):672–674. doi: 10.1002/ppul.24319. [DOI] [PubMed] [Google Scholar]
- 15.Muirhead C, Verzasconi D, Joshi S. At-home compounding preparation of slow desensitization of elexacaftor/tezacaftor/ivacaftor for delayed hypersensitivity rash. Pediatr Pulmonol . 2022;57(7):1779–1781. doi: 10.1002/ppul.25938. [DOI] [PubMed] [Google Scholar]


