Figure 5: Cellular Mechanisms of Na+ and K+ Transport in the Aldosterone-sensitive Distal Nephron (ASDN).
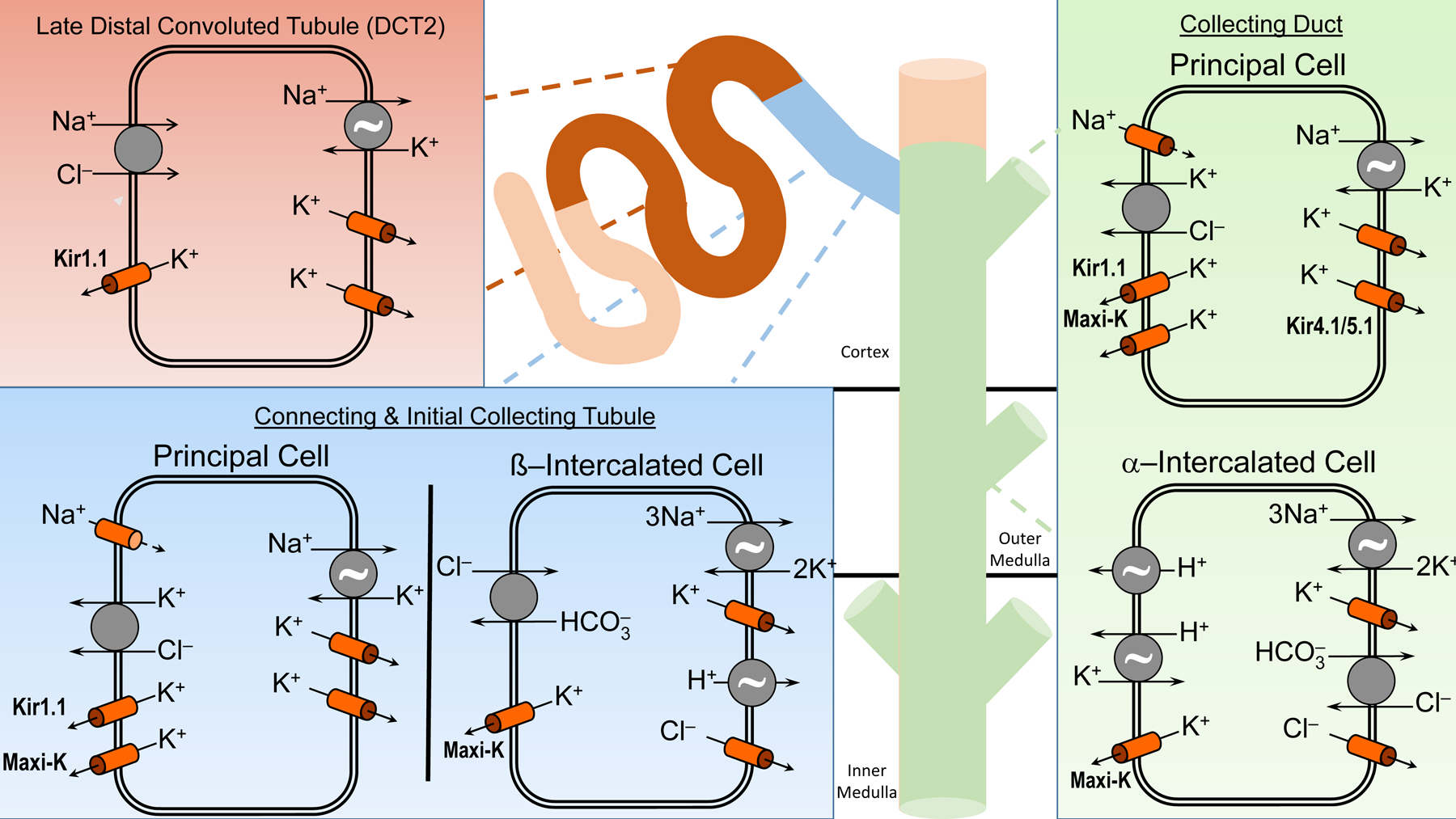
The ASDN, consisting of the late distal convoluted tubule (DCT2), the connecting (CNT) and initial collecting tubule (ICT), and the collecting duct (CD), express the mineralocorticoid receptor (MR) and the high affinity enzyme 11ß-hydroxysteroid dehydrogenase type 2 (11ß-HSD2) which oxidizes cortisol to cortisone and is important in conferring mineralocorticoid specificity to the ASDN. Na+ reabsorption occurs predominantly by the electroneutral NaCl cotransporter in the DCT2, with progressive increasing the proportion of electrogenic Na+ absorption occurring in the CNT, ICT and CD. K+ in the ASDN is secreted by two classes of K+ channels, inwardly-rectifying K+ channels (Kir1.1; also known as the renal outer medullary channel or ROMK) and large conductance Ca2+-activated K+ channels (also known as BK or Maxi-K channels). The apical KCl cotransporter in principal cells is involved in non-conductive K+ secretion. K+ absorption is an active process driven by an apical HKα1 H+K+-ATPase and basolateral K+ channels in intercalated cells and in the principal cells by HKα2 H+K+-ATPase (not shown). The ~ symbol indicates an ATPase.
