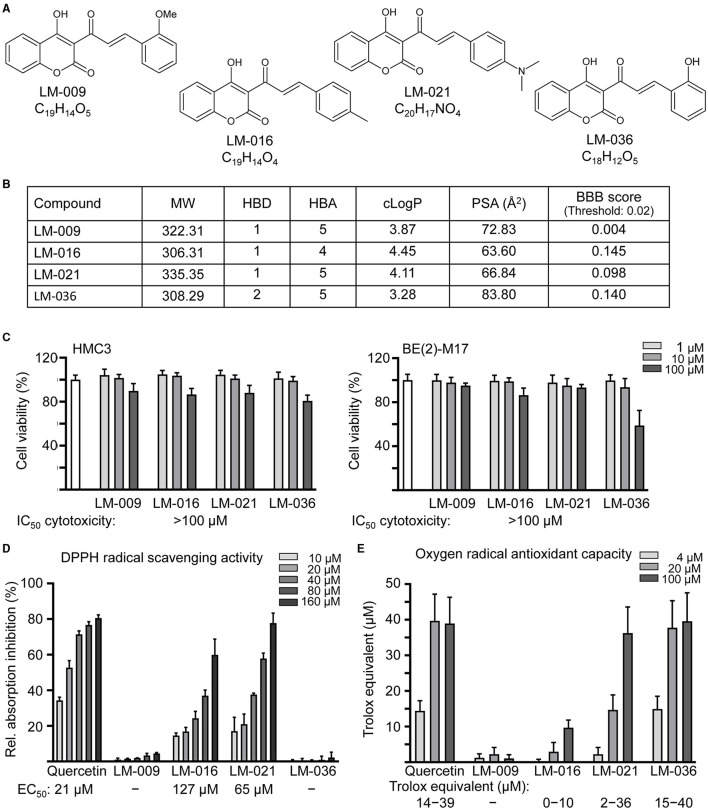
Figure 1.
LM compounds. (A) Structure and formula of LM-009,−016,−021, and−036. (B) Molecular weight (MW), hydrogen bond donor (HBD), hydrogen bond acceptor (HBA), calculated octanol-water partition coefficient (cLogP), polar surface area (PSA), and blood–brain barrier (BBB) permeation score of LM compounds. (C) Cytotoxicity of LM compounds against human HMC3 and BE(2)-M17 cells examined by MTT assay. Cells were treated with LM compound (1–100 μM) and cell viability was measured the next day (n = 3). To normalize, the relative viability of untreated cells was set at 100%. Shown below were cytotoxicity IC50 values. (D) DPPH free radical scavenging activity (10–160 μM) and (E) oxygen radical absorbance capacity (4–100 μM) of LM compounds and quercetin (as a positive control; n = 3). Shown below were EC50 or trolox equivalent values.