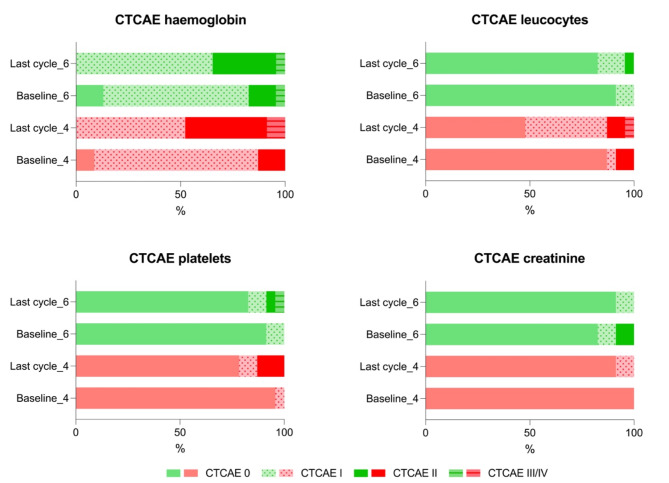
Fig. 3.
Adverse events based on CTCAE v. 5.0. Adverse events based on CTCAE v. 5.0 for haemoglobin, leukocytes, platelets, and creatinine were compared in percentages between patients undergoing 4-week (red) and 6-week (green) treatment intervals, both at baseline and the final treatment cycle. Grade ≥ III toxicities are indicated by horizontal lines. From baseline, in the 4-week treatment interval group, there were two instances of grade III anemia and one of grade III leukopenia. In the 6-week treatment interval group, one grade III anemia and one grade III thrombocytopenia were observed; however, the same patient already had grade III anemia at baseline. There were no other grade III/IV toxicities in either group