Abstract
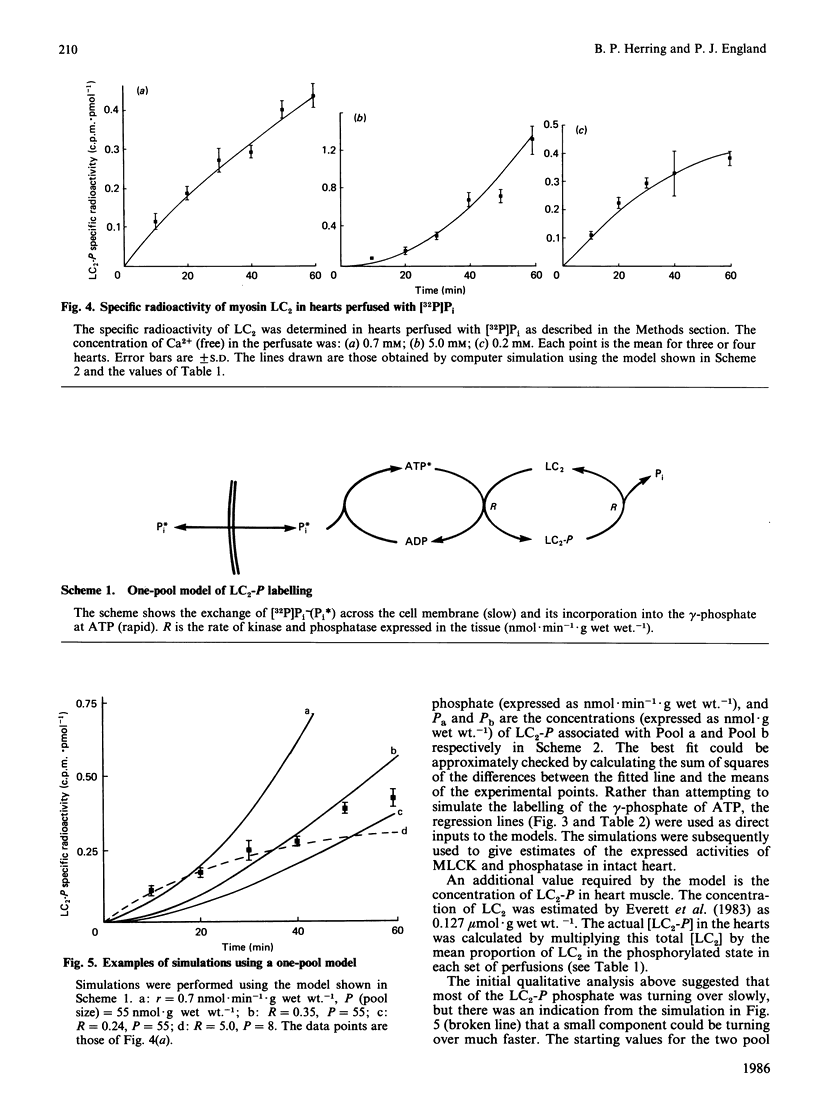
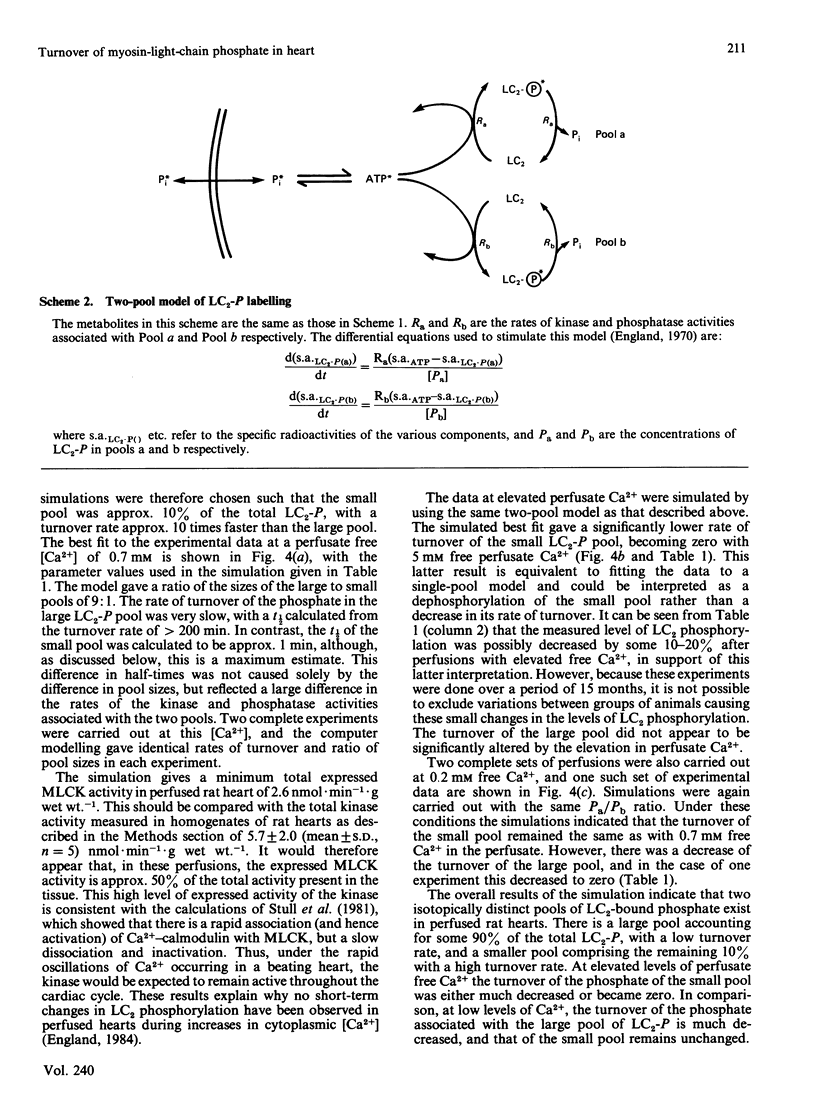
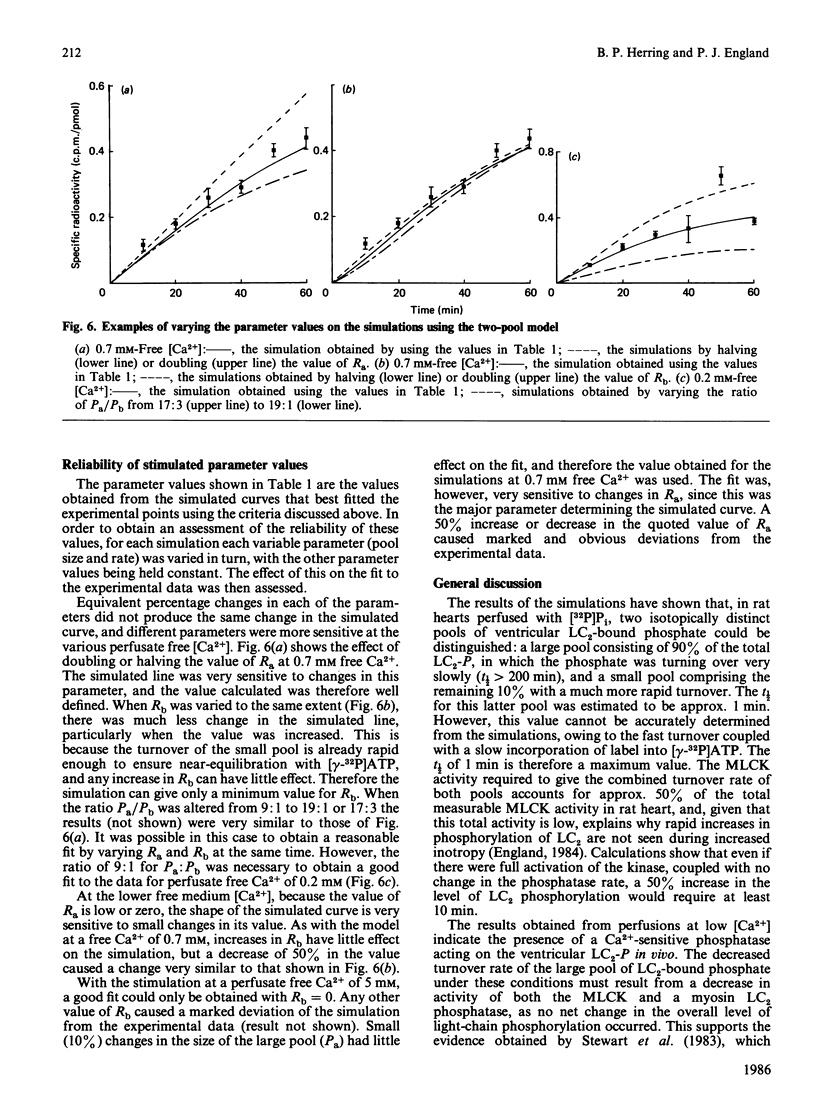
The rate of exchange of phosphate bound to ventricular myosin light chain-2 (LC2-P) was measured in rat hearts perfused with [32P]Pi at various levels of perfusate Ca2+. Computer simulations of the light-chain labelling suggested the presence of two isotopically distinct pools of LC2-P, one large pool comprising 90% of the total and a small pool consisting of the remaining 10%. At control levels of perfusate Ca2+ the phosphate of the large pool turned over very slowly (t 1/2 congruent to 250 min), whereas that of the small pool turned over much more rapidly (t 1/2 congruent to 1 min). At high levels of perfusate free Ca2+ (5mM) the turnover of the phosphate of the small pool decreased markedly, whereas that of the large pool remained little changed. Conversely, at low perfusate free Ca2+ (0.2 mM), the turnover of the large pool decreased, whereas that of the small pool remained unchanged. The possible identity of these two pools is discussed. The total myosin-light-chain kinase activity of rat ventricle was found to be only 2-3-fold higher than the kinase activity expressed in the heart under control conditions. This, coupled with the very low turnover of most of the LC2-bound phosphate, implies that, in heart, there is insufficient myosin-light-chain kinase activity to cause a rapid rise in the overall level of light-chain phosphorylation, even under conditions of increased cytoplasmic Ca2+.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barsotti R. J., Butler T. M. Chemical energy usage and myosin light chain phosphorylation in mammalian skeletal muscle. J Muscle Res Cell Motil. 1984 Feb;5(1):45–64. doi: 10.1007/BF00713151. [DOI] [PubMed] [Google Scholar]
- Butler T. M., Siegman M. J., Mooers S. U., Barsotti R. J. Myosin light chain phosphorylation does not modulate cross-bridge cycling rate in mouse skeletal muscle. Science. 1983 Jun 10;220(4602):1167–1169. doi: 10.1126/science.6857239. [DOI] [PubMed] [Google Scholar]
- Chang J. Y., Knecht R., Braun D. G. Amino acid analysis in the picomole range by precolumn derivatization and high-performance liquid chromatography. Methods Enzymol. 1983;91:41–48. doi: 10.1016/s0076-6879(83)91009-1. [DOI] [PubMed] [Google Scholar]
- Cohen P. The subunit structure of rabbit-skeletal-muscle phosphorylase kinase, and the molecular basis of its activation reactions. Eur J Biochem. 1973 Apr 2;34(1):1–14. doi: 10.1111/j.1432-1033.1973.tb02721.x. [DOI] [PubMed] [Google Scholar]
- Cole H. A., Griffiths H. S., Patchell V. B., Perry S. V. Two-site phosphorylation of the phosphorylatable light chain (20-kDa light chain) of chicken gizzard myosin. FEBS Lett. 1985 Jan 28;180(2):165–169. doi: 10.1016/0014-5793(85)81064-4. [DOI] [PubMed] [Google Scholar]
- Cooke R., Franks K., Stull J. T. Myosin phosphorylation regulates the ATPase activity of permeable skeletal muscle fibers. FEBS Lett. 1982 Jul 19;144(1):33–37. doi: 10.1016/0014-5793(82)80563-2. [DOI] [PubMed] [Google Scholar]
- Crow M. T., Kushmerick M. J. Phosphorylation of myosin light chains in mouse fast-twitch muscle associated with reduced actomyosin turnover rate. Science. 1982 Aug 27;217(4562):835–837. doi: 10.1126/science.6285472. [DOI] [PubMed] [Google Scholar]
- England P. J. Computer simulation of the non-steady-state radioactive labelling of a system of metabolite pools with constant rates of influx and efflux. Biochem J. 1970 May;117(4):697–698. doi: 10.1042/bj1170697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- England P. J. The significance of phosphorylation of myosin light chains in heart. J Mol Cell Cardiol. 1984 Jul;16(7):591–595. doi: 10.1016/s0022-2828(84)80623-9. [DOI] [PubMed] [Google Scholar]
- England P. J., Walsh D. A. A rapid method for the measurement of [gamma-32P]ATP specific radioactivity in tissue extracts and its application to the study of 32Pi uptake in perfused rat heart. Anal Biochem. 1976 Oct;75(2):429–435. doi: 10.1016/0003-2697(76)90096-8. [DOI] [PubMed] [Google Scholar]
- Everett A. W., Prior G., Clark W. A., Zak R. Quantitation of myosin in muscle. Anal Biochem. 1983 Apr 1;130(1):102–107. doi: 10.1016/0003-2697(83)90655-3. [DOI] [PubMed] [Google Scholar]
- FISCHER E. H., KREBS E. G. The isolation and crystallization of rabbit skeletal muscle phosphorylase b. J Biol Chem. 1958 Mar;231(1):65–71. [PubMed] [Google Scholar]
- Franks K., Cooke R., Stull J. T. Myosin phosphorylation decreases the ATPase activity of cardiac myofibrils. J Mol Cell Cardiol. 1984 Jul;16(7):597–604. doi: 10.1016/s0022-2828(84)80624-0. [DOI] [PubMed] [Google Scholar]
- Frearson N., Perry S. V. Phosphorylation of the light-chain components of myosin from cardiac and red skeletal muscles. Biochem J. 1975 Oct;151(1):99–107. doi: 10.1042/bj1510099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furukawa K. I., Ikebe M., Inoue A., Tonomura Y. The amount of nucleotide binding and the P1-burst size of myosin adenosinetriphosphatase: evidence for the nonidentical two-headed structure of myosin. J Biochem. 1980 Dec;88(6):1629–1641. doi: 10.1093/oxfordjournals.jbchem.a133139. [DOI] [PubMed] [Google Scholar]
- High C. W., Stull J. T. Phosphorylation of myosin in perfused rabbit and rat hearts. Am J Physiol. 1980 Dec;239(6):H756–H764. doi: 10.1152/ajpheart.1980.239.6.H756. [DOI] [PubMed] [Google Scholar]
- Hoh J. F., McGrath P. A., Hale P. T. Electrophoretic analysis of multiple forms of rat cardiac myosin: effects of hypophysectomy and thyroxine replacement. J Mol Cell Cardiol. 1978 Nov;10(11):1053–1076. doi: 10.1016/0022-2828(78)90401-7. [DOI] [PubMed] [Google Scholar]
- Holroyde M. J., Small D. A., Howe E., Solaro R. J. Isolation of cardiac myofibrils and myosin light chains with in vivo levels of light chain phosphorylation. Biochim Biophys Acta. 1979 Nov 1;587(4):628–637. doi: 10.1016/0304-4165(79)90014-x. [DOI] [PubMed] [Google Scholar]
- Ikebe M., Hartshorne D. J. Phosphorylation of smooth muscle myosin at two distinct sites by myosin light chain kinase. J Biol Chem. 1985 Aug 25;260(18):10027–10031. [PubMed] [Google Scholar]
- Jeacocke S. A., England P. J. Phosphorylation of myosin light chains in perfused rat heart. Effect of adrenaline and increased cytoplasmic calcium ions. Biochem J. 1980 Jun 15;188(3):763–768. doi: 10.1042/bj1880763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamm K. E., Stull J. T. The function of myosin and myosin light chain kinase phosphorylation in smooth muscle. Annu Rev Pharmacol Toxicol. 1985;25:593–620. doi: 10.1146/annurev.pa.25.040185.003113. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Manning D. R., Stull J. T. Myosin light chain phosphorylation and phosphorylase A activity in rat extensor digitorum longus muscle. Biochem Biophys Res Commun. 1979 Sep 12;90(1):164–170. doi: 10.1016/0006-291x(79)91604-8. [DOI] [PubMed] [Google Scholar]
- Morano I., Hofmann F., Zimmer M., Rüegg J. C. The influence of P-light chain phosphorylation by myosin light chain kinase on the calcium sensitivity of chemically skinned heart fibres. FEBS Lett. 1985 Sep 23;189(2):221–224. doi: 10.1016/0014-5793(85)81027-9. [DOI] [PubMed] [Google Scholar]
- Murray K. J., England P. J. Contraction in intact pig aortic strips is not always associated with phosphorylation of myosin light chains. Biochem J. 1980 Dec 15;192(3):967–970. doi: 10.1042/bj1920967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrie W. T., Perry S. V. An electrophoretic study of the low-molecular-weight components of myosin. Biochem J. 1970 Aug;119(1):31–38. doi: 10.1042/bj1190031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrie W. T., Smillie L. B., Perry S. B. A phosphorylated light-chain component of myosin from skeletal muscle. Biochem J. 1973 Sep;135(1):151–164. doi: 10.1042/bj1350151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pires E., Perry S. V., Thomas M. A. Myosin light-chain kinase, a new enzyme from striated muscle. FEBS Lett. 1974 May 1;41(2):292–296. doi: 10.1016/0014-5793(74)81232-9. [DOI] [PubMed] [Google Scholar]
- Silver P. J., Stull J. T. Quantitation of myosin light chain phosphorylation in small tissue samples. J Biol Chem. 1982 Jun 10;257(11):6137–6144. [PubMed] [Google Scholar]
- Stewart A. A., Ingebritsen T. S., Cohen P. The protein phosphatases involved in cellular regulation. 5. Purification and properties of a Ca2+/calmodulin-dependent protein phosphatase (2B) from rabbit skeletal muscle. Eur J Biochem. 1983 May 2;132(2):289–295. doi: 10.1111/j.1432-1033.1983.tb07361.x. [DOI] [PubMed] [Google Scholar]
- Sweeney H. L., Kushmerick M. J. Myosin phosphorylation in permeabilized rabbit psoas fibers. Am J Physiol. 1985 Sep;249(3 Pt 1):C362–C365. doi: 10.1152/ajpcell.1985.249.3.C362. [DOI] [PubMed] [Google Scholar]
- Trybus K. M., Lowey S. Mechanism of smooth muscle myosin phosphorylation. J Biol Chem. 1985 Dec 15;260(29):15988–15995. [PubMed] [Google Scholar]
- WOLLENBERGER A., RISTAU O., SCHOFFA G. [A simple technic for extremely rapid freezing of large pieces of tissue]. Pflugers Arch Gesamte Physiol Menschen Tiere. 1960;270:399–412. [PubMed] [Google Scholar]
- Walsh M. P., Cavadore J. C., Vallet B., Demaille J. G. Calmodulin-dependent myosin light chain kinases from cardiac and smooth muscle: a comparative study. Can J Biochem. 1980 Apr;58(4):299–308. doi: 10.1139/o80-040. [DOI] [PubMed] [Google Scholar]
- Walsh M. P., Vallet B., Autric F., Demaille J. G. Purification and characterization of bovine cardiac calmodulin-dependent myosin light chain kinase. J Biol Chem. 1979 Dec 10;254(23):12136–12144. [PubMed] [Google Scholar]
- Westwood S. A., Perry S. V. The effect of adrenaline on the phosphorylation of the P light chain of myosin and troponin I in the perfused rabbit heart. Biochem J. 1981 Jul 1;197(1):185–193. doi: 10.1042/bj1970185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- d'Albis A., Pantaloni C., Bechet J. J. An electrophoretic study of native myosin isozymes and of their subunit content. Eur J Biochem. 1979 Sep;99(2):261–272. doi: 10.1111/j.1432-1033.1979.tb13253.x. [DOI] [PubMed] [Google Scholar]


