Figure 1.
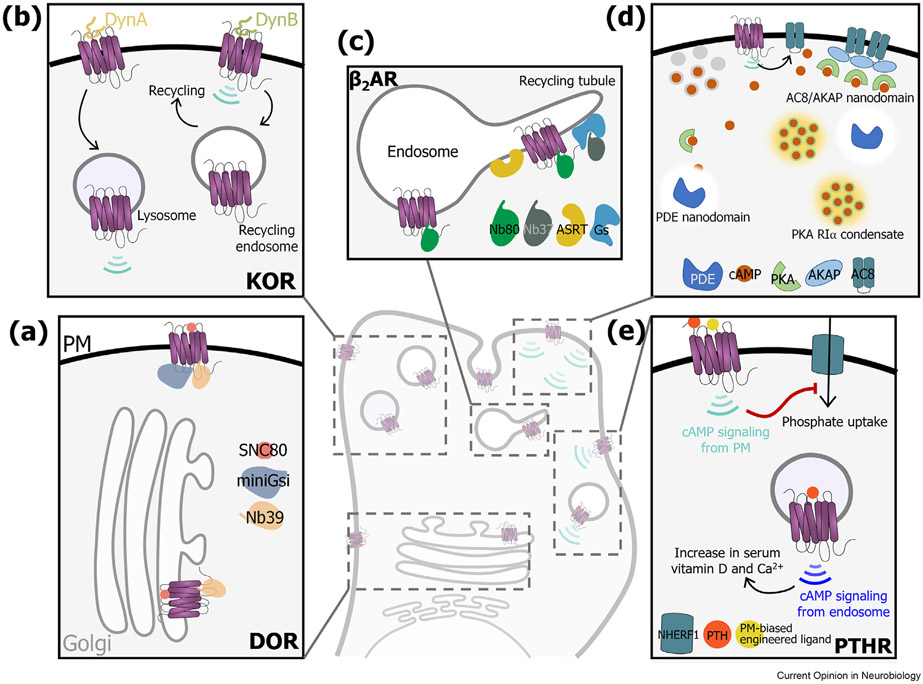
Diversity in location, interaction, signaling, and functional outcomes in GPCRs. GPCRs exhibit enormous diversity in their localization and interaction profiles, and consequently generate spatiotemporally distinct signaling outcomes. This figure highlights a few examples. (a) The subcellular location of DOR determines receptor active states. Although receptors at both the Golgi and the plasma membrane (PM) inhibit cAMP upon activation by a cell permeable agonist, receptors at the PM recruit Nb39 (biosensor for active DOR) as well as miniGsi (biosensor for Gi binding to active DOR), but only Nb39 at the Golgi. (b) KOR signaling is selectively modulated by different dynorphin peptides in a compartment-specific manner. DynA sorts KOR to the lysosomal pathway and induces sustained cAMP signaling from lysosomal compartments, while DynB drives recycling of KOR without activating receptors on the endosome. Therefore, DynA predominantly drives signaling from endosomes, while DynB predominantly drives signaling from the surface. (c) Sub-organellar microdomains accessed by β2AR allow distinct spatiotemporal control over signaling. Active β2AR (marked by Nb80) is present on bulk as well as receptor C-terminal sequence-dependent recycling tubules marked by actin/sorting nexin/retromer tubular (ASRT) microdomains. However, the receptor stimulates Gs (visualized by Nb37) only on ASRT microdomains. PKA-driven localization of β2AR on ASRT domains biases downstream transcriptional responses to genes controlled by signals from endosomes. (d) Multiple mechanisms operate to maintain spatiotemporally confined responses from the highly dynamic second messenger cAMP. In an unstimulated state, most of the cAMP is sequestered allowing the generation of cAMP-free nanodomains around phosphodiesterase (PDE) which ‘protect’ cAMP effectors. Upon stimulation the high concentrations of cAMP saturate their binding sites and ultimately deplete the ‘protected’ zone around PDE thereby exposing cAMP effectors. Parallelly, under stimulated conditions, cAMP is sequestered by the RIa subunit of PKA in biomolecular condensates formed by liquid-liquid phase separation. In oscillatory circuits triggered by Ca2+, the relative phase of cAMP and Ca2+ are maintained by the formation of adenylyl cyclase 8 (AC8) and PKA recruiting A-kinase anchoring protein (AKAP) nanoclusters on the plasma membrane. (e) Activation of the parathyroid hormone receptor type I (PTHR) triggers distinct cAMP mediated physiological responses based on receptor localization. Prior to endocytosis, transient activation of the receptor on the PM leads to Na+/H+ exchanger regulatory factor-1 (NHERF1)-dependent reduction in serum phosphate levels. On the other hand, sustained activation of the receptor in endosomes upon endocytosis leads to increase in serum vitamin D and Ca2+ levels. Unlike PTH that activates PTHR at both locations with a transient PM response and a sustained endosomal response, a PM-biased ligand could generate sustained cAMP signals from the plasma membrane thus biasing the physiological outcome.