Abstract
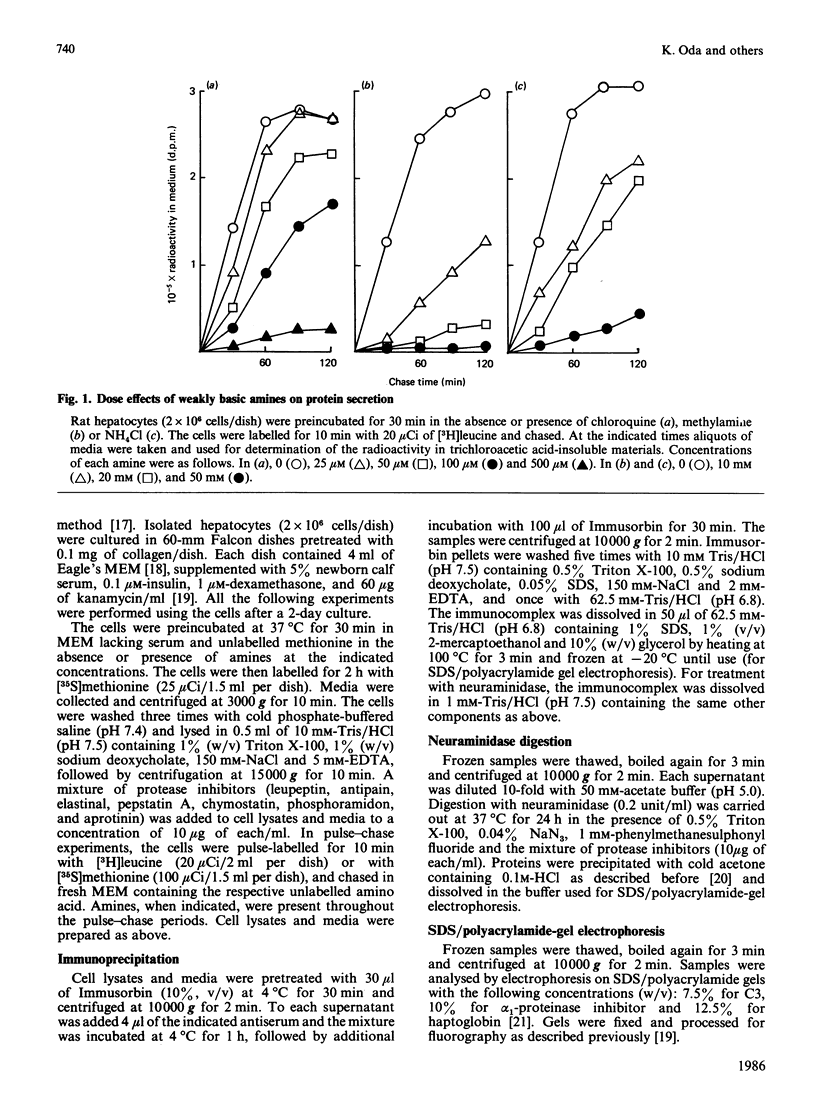
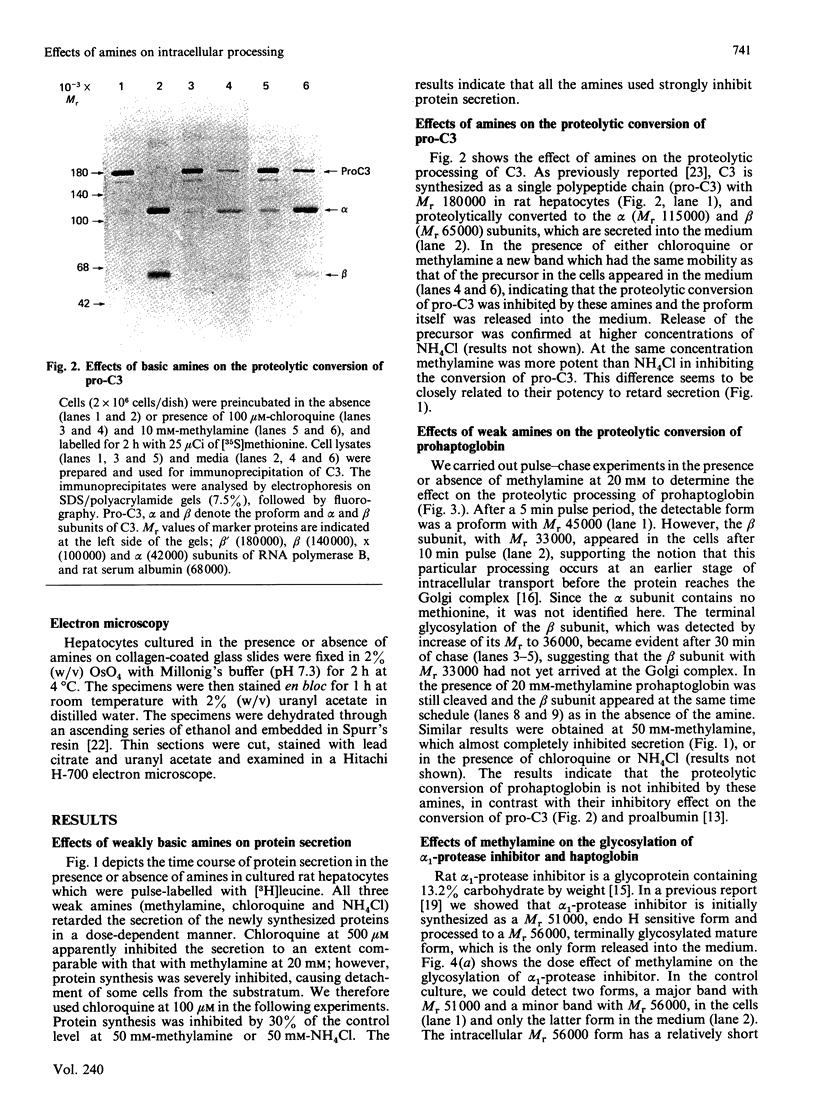
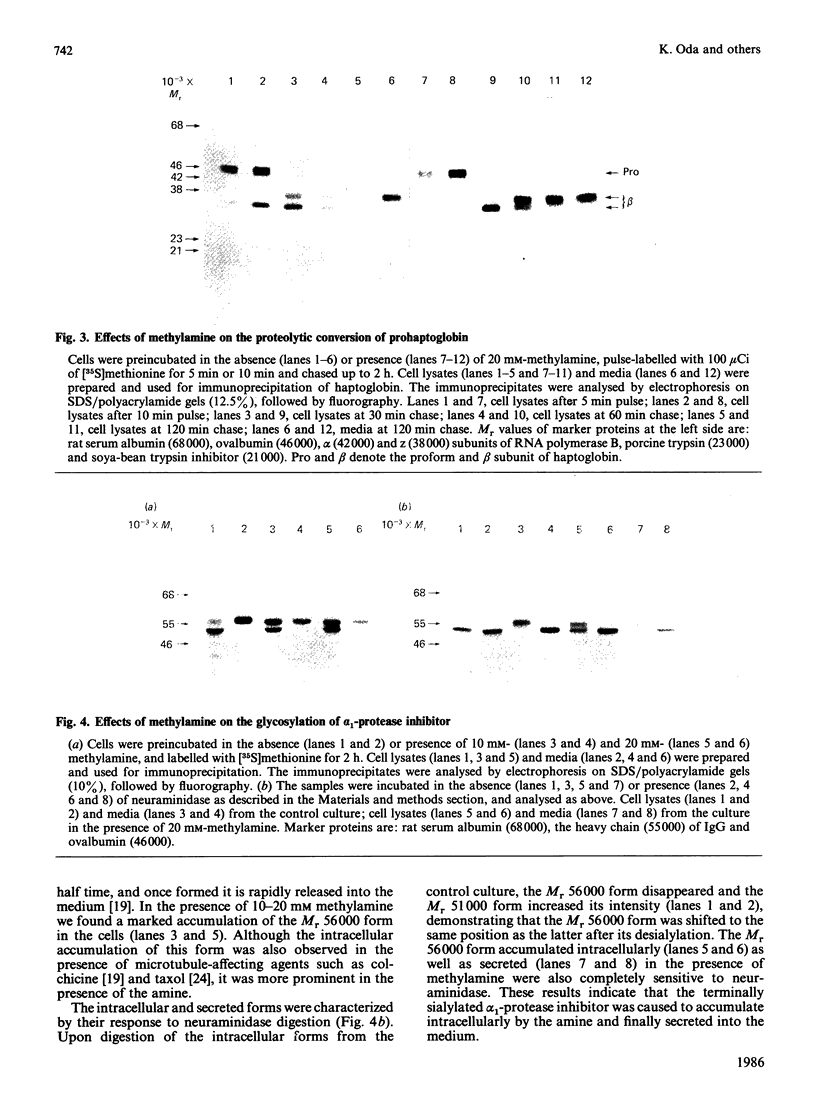
We examined the effects of weakly basic amines on the secretion and post-translational modifications of secretory proteins in cultured rat hepatocytes. Weakly basic amines such as methylamine, chloroquine and NH4Cl strongly inhibited not only protein secretion, but also the proteolytic conversion of a proform of complement C3, allowing the precursor to be released into the medium. The amines, however, had no effect on the proteolytic conversion of prohaptoglobin into its subunits. Since available evidence indicates that the conversion of pro-C3 occurs at the Golgi complex while that of prohaptoglobin takes place in the endoplasmic reticulum, it is most likely that the weak bases specifically affect the proteolytic event occurring at the Golgi complex. Electron microscopic observations confirmed that the amines caused morphological changes of the Golgi complex, consisting of dilated cisternae and swollen vacuoles. When the glycosylation of alpha 1-protease inhibitor and haptoglobin was examined, it was found that the amines caused a marked accumulation in the cells of both glycoproteins corresponding to the mature secreted forms. Neuraminidase digestion demonstrated that the glycoproteins accumulating in response to the amines had acquired terminal sialic acid. The results indicate that the amines do not significantly affect terminal glycosylation, in contrast with their definite effect on proteolytic processing, despite the fact that both modifications take place in the Golgi complex.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson R. G., Pathak R. K. Vesicles and cisternae in the trans Golgi apparatus of human fibroblasts are acidic compartments. Cell. 1985 Mar;40(3):635–643. doi: 10.1016/0092-8674(85)90212-0. [DOI] [PubMed] [Google Scholar]
- Barr R., Safranski K., Sun I. L., Crane F. L., Morré D. J. An electrogenic proton pump associated with the Golgi apparatus of mouse liver driven by NADH and ATP. J Biol Chem. 1984 Nov 25;259(22):14064–14067. [PubMed] [Google Scholar]
- Dean R. T., Jessup W., Roberts C. R. Effects of exogenous amines on mammalian cells, with particular reference to membrane flow. Biochem J. 1984 Jan 1;217(1):27–40. doi: 10.1042/bj2170027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domdey H., Wiebauer K., Kazmaier M., Müller V., Odink K., Fey G. Characterization of the mRNA and cloned cDNA specifying the third component of mouse complement. Proc Natl Acad Sci U S A. 1982 Dec;79(24):7619–7623. doi: 10.1073/pnas.79.24.7619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forgac M., Cantley L., Wiedenmann B., Altstiel L., Branton D. Clathrin-coated vesicles contain an ATP-dependent proton pump. Proc Natl Acad Sci U S A. 1983 Mar;80(5):1300–1303. doi: 10.1073/pnas.80.5.1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galloway C. J., Dean G. E., Marsh M., Rudnick G., Mellman I. Acidification of macrophage and fibroblast endocytic vesicles in vitro. Proc Natl Acad Sci U S A. 1983 Jun;80(11):3334–3338. doi: 10.1073/pnas.80.11.3334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glickman J., Croen K., Kelly S., Al-Awqati Q. Golgi membranes contain an electrogenic H+ pump in parallel to a chloride conductance. J Cell Biol. 1983 Oct;97(4):1303–1308. doi: 10.1083/jcb.97.4.1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikehara Y., Miyasato M., Ogata S., Oda K. Multiple forms of rat-serum alpha 1-protease inhibitor. Involvement of sialic acid in the multiplicity of three original forms. Eur J Biochem. 1981 Apr;115(2):253–260. doi: 10.1111/j.1432-1033.1981.tb05231.x. [DOI] [PubMed] [Google Scholar]
- Ikehara Y., Oda K., Kato K. Conversion of proalbumin into serum albumin in the secretory vesicles of rat liver. Biochem Biophys Res Commun. 1976 Sep 7;72(1):319–326. doi: 10.1016/0006-291x(76)90996-7. [DOI] [PubMed] [Google Scholar]
- Kominami T., Oda K., Ikehara Y. Induction of rat hepatic alkaline phosphatase and its appearance in serum: electrophoretic characterization of liver-membranous and serum-soluble forms. J Biochem. 1984 Sep;96(3):901–911. doi: 10.1093/oxfordjournals.jbchem.a134909. [DOI] [PubMed] [Google Scholar]
- Kornfeld R., Kornfeld S. Assembly of asparagine-linked oligosaccharides. Annu Rev Biochem. 1985;54:631–664. doi: 10.1146/annurev.bi.54.070185.003215. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Misumi Y., Takami N., Ikehara Y. Biosynthesis and processing of pro-C3, a precursor of the third component of complement in rat hepatocytes: effect of secretion-blocking agents. FEBS Lett. 1984 Sep 17;175(1):63–67. doi: 10.1016/0014-5793(84)80570-0. [DOI] [PubMed] [Google Scholar]
- Misumi Y., Tanaka Y., Ikehara Y. Biosynthesis, intracellular processing and secretion of haptoglobin in cultured rat hepatocytes. Biochem Biophys Res Commun. 1983 Jul 29;114(2):729–736. doi: 10.1016/0006-291x(83)90841-0. [DOI] [PubMed] [Google Scholar]
- Oda K., Ikehara Y., Kato K. Multiple forms of albumin and their conversion from pro-type to serum-type in rat liver in vivo. Biochim Biophys Acta. 1978 Sep 26;536(1):97–105. doi: 10.1016/0005-2795(78)90055-7. [DOI] [PubMed] [Google Scholar]
- Oda K., Ikehara Y. Monensin inhibits the conversion of proalbumin to serum albumin in cultured rat hepatocytes. Biochem Biophys Res Commun. 1982 Mar 30;105(2):766–772. doi: 10.1016/0006-291x(82)91500-5. [DOI] [PubMed] [Google Scholar]
- Oda K., Ikehara Y. Taxol, a potent promoter of microtubule assembly, inhibits secretion of plasma proteins in cultured rat hepatocytes. Biochem Biophys Res Commun. 1982 Jul 30;107(2):561–567. doi: 10.1016/0006-291x(82)91528-5. [DOI] [PubMed] [Google Scholar]
- Oda K., Ikehara Y. Weakly basic amines inhibit the proteolytic conversion of proalbumin to serum albumin in cultured rat hepatocytes. Eur J Biochem. 1985 Nov 4;152(3):605–609. doi: 10.1111/j.1432-1033.1985.tb09238.x. [DOI] [PubMed] [Google Scholar]
- Oda K., Misumi Y., Ikehara Y. Disparate effects of monensin and colchicine on intracellular processing of secretory proteins in cultured rat hepatocytes. Eur J Biochem. 1983 Sep 15;135(2):209–216. doi: 10.1111/j.1432-1033.1983.tb07639.x. [DOI] [PubMed] [Google Scholar]
- Ohkuma S., Moriyama Y., Takano T. Identification and characterization of a proton pump on lysosomes by fluorescein-isothiocyanate-dextran fluorescence. Proc Natl Acad Sci U S A. 1982 May;79(9):2758–2762. doi: 10.1073/pnas.79.9.2758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redman C. M., Cherian M. G. The secretory pathways of rat serum glycoproteins and albumin. Localization of newly formed proteins within the endoplasmic reticulum. J Cell Biol. 1972 Feb;52(2):231–245. doi: 10.1083/jcb.52.2.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth J., Taatjes D. J., Lucocq J. M., Weinstein J., Paulson J. C. Demonstration of an extensive trans-tubular network continuous with the Golgi apparatus stack that may function in glycosylation. Cell. 1985 Nov;43(1):287–295. doi: 10.1016/0092-8674(85)90034-0. [DOI] [PubMed] [Google Scholar]
- Schneider D. L. ATP-dependent acidification of intact and disrupted lysosomes. Evidence for an ATP-driven proton pump. J Biol Chem. 1981 Apr 25;256(8):3858–3864. [PubMed] [Google Scholar]
- Schwartz A. L., Strous G. J., Slot J. W., Geuze H. J. Immunoelectron microscopic localization of acidic intracellular compartments in hepatoma cells. EMBO J. 1985 Apr;4(4):899–904. doi: 10.1002/j.1460-2075.1985.tb03716.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seglen P. O. Preparation of isolated rat liver cells. Methods Cell Biol. 1976;13:29–83. doi: 10.1016/s0091-679x(08)61797-5. [DOI] [PubMed] [Google Scholar]
- Spurr A. R. A low-viscosity epoxy resin embedding medium for electron microscopy. J Ultrastruct Res. 1969 Jan;26(1):31–43. doi: 10.1016/s0022-5320(69)90033-1. [DOI] [PubMed] [Google Scholar]
- Stone D. K., Xie X. S., Racker E. An ATP-driven proton pump in clathrin-coated vesicles. J Biol Chem. 1983 Apr 10;258(7):4059–4062. [PubMed] [Google Scholar]
- Strous G. J., Du Maine A., Zijderhand-Bleekemolen J. E., Slot J. W., Schwartz A. L. Effect of lysosomotropic amines on the secretory pathway and on the recycling of the asialoglycoprotein receptor in human hepatoma cells. J Cell Biol. 1985 Aug;101(2):531–539. doi: 10.1083/jcb.101.2.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wibo M., Poole B. Protein degradation in cultured cells. II. The uptake of chloroquine by rat fibroblasts and the inhibition of cellular protein degradation and cathepsin B1. J Cell Biol. 1974 Nov;63(2 Pt 1):430–440. doi: 10.1083/jcb.63.2.430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashiro D. J., Fluss S. R., Maxfield F. R. Acidification of endocytic vesicles by an ATP-dependent proton pump. J Cell Biol. 1983 Sep;97(3):929–934. doi: 10.1083/jcb.97.3.929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang F., Schneider D. L. The bioenergetics of Golgi apparatus function: evidence for an ATP-dependent proton pump. Biochem Biophys Res Commun. 1983 Jul 29;114(2):620–625. doi: 10.1016/0006-291x(83)90825-2. [DOI] [PubMed] [Google Scholar]
- de Duve C. Lysosomes revisited. Eur J Biochem. 1983 Dec 15;137(3):391–397. doi: 10.1111/j.1432-1033.1983.tb07841.x. [DOI] [PubMed] [Google Scholar]
- de Duve C., de Barsy T., Poole B., Trouet A., Tulkens P., Van Hoof F. Commentary. Lysosomotropic agents. Biochem Pharmacol. 1974 Sep 15;23(18):2495–2531. doi: 10.1016/0006-2952(74)90174-9. [DOI] [PubMed] [Google Scholar]