Figure 3.
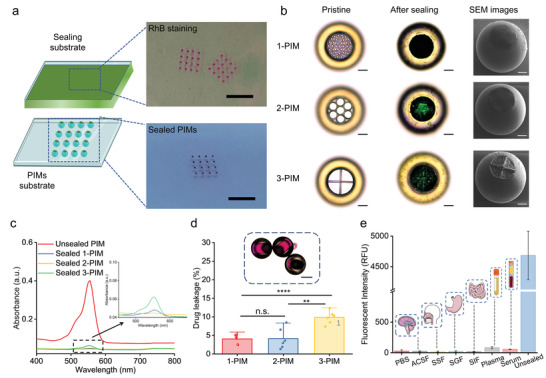
Drug protection ability of the microrobotic system. a) Schematic of the mechanically controlled sealing process (left). The sealing material was first spin‐coated into a uniform film and then rapidly brought into contact with the top surface of the microrobot. Subsequent solidification of the sealing material resulted in a precisely formed thin sealing cap on the microrobot. Images (right) show the physical transfer of RhB staining on the sealing substrate (top) and the sealed PIMs (bottom). Scale bars = 5 mm. b) Images of 1‐PIM, 2‐PIM, and 3‐PIM (top to bottom) before and after sealing. From left to right: bright‐field microscopy images of PIMs before sealing (left), bright‐field microscopy images of PIMs after sealing (middle), and SEM images of PIMs after sealing (right). Scale bars = 50 µm. c) UV–Vis spectra quantifying RhB leakage in incubation solution using PIMs with or without sealing layers. The inset figure shows the UV–Vis spectra of sealed PIMs. d) Drug leakage of 1‐PIM, 2‐PIM, and 3‐PIM designs. All values are expressed as mean ± SD, n = 6, significance determined by an unpaired, two‐tailed t‐test, Student's t‐test, p = 0.929146 for 1‐PIMs versus 2‐PIMs, **** p = 0.000051 for 1‐PIMs versus 3‐PIMs, ** p = 0.001655 for 2‐PIMs versus 3‐PIMs. The inset optical image shows no RhB leakage from sealed 2‐PIMs. Scale bar = 100 µm. e) Fluorescence intensity of RhB in different biofluids after incubating with sealed 2‐PIMs. All values are expressed as mean ± SD, n = 3. From left to right: phosphate‐buffered saline (PBS), artificial cerebrospinal fluid (ACSF), simulated salivary fluid (SSF), simulated gastric fluid (SGF), simulated intestinal fluid (SIF), human plasma (Plasma), horse serum (Serum). Positive control: unsealed 2‐PIM in PBS (Unsealed).
