Abstract
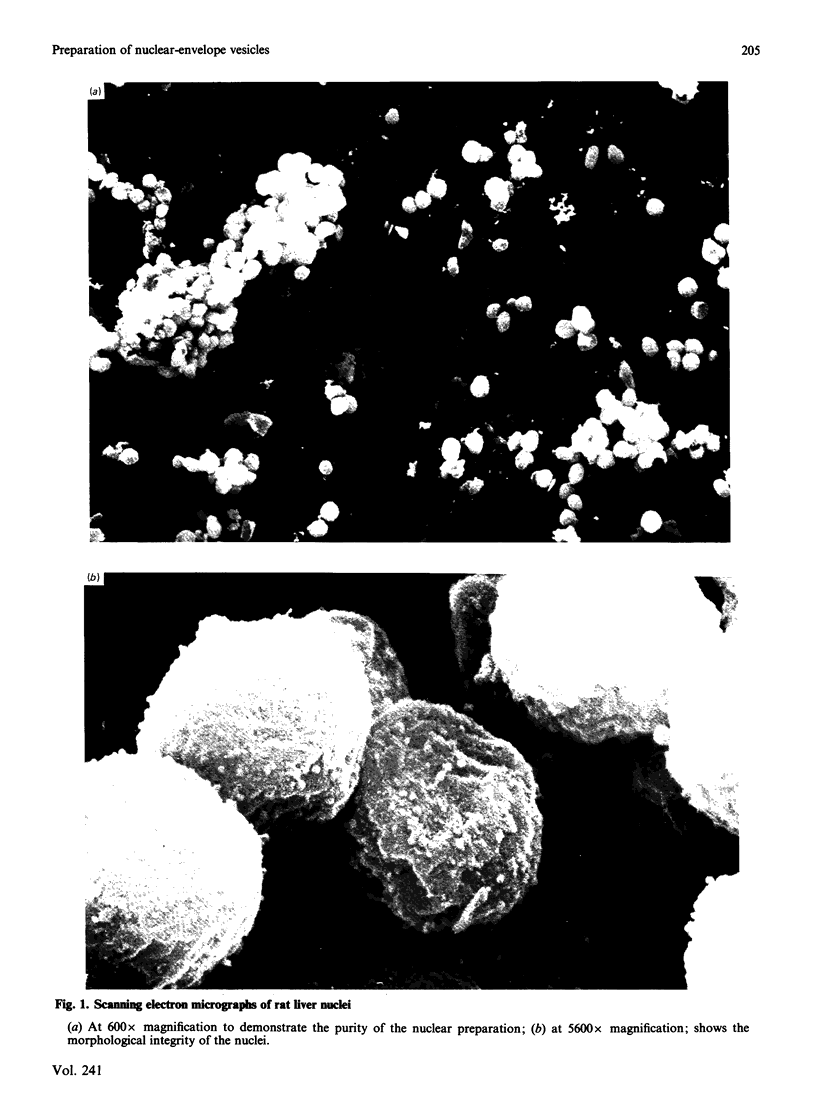
We describe a procedure for the preparation of sealed nuclear-envelope vesicles from rat liver nuclei. These vesicles are strikingly similar in their polypeptide composition when compared with those of nuclear envelopes prepared conventionally using deoxyribonuclease I. Subfractionation analysis by means of extraction with high salt and urea show that the components of the nuclear envelope, e.g. the pore-complex/lamina fraction, are present. The residual DNA content is only 1.5%, and typical preparations consist of about 80% vesicles, with the vesicular character of these envelopes shown by microscopic and biochemical studies. The vesicles can be obtained in high yield, are tight and stable for at least two days and are enriched in a nucleoside triphosphatase thought to be involved in nucleocytoplasmic transport processes. Because the vesicles are largely free of components of the nuclear interior, but retain properties of intact nuclei, we believe that they are a valuable model system to study nucleocytoplasmic transport. Although in transport studies with isolated nuclei interference from intranuclear events has to be considered, the nuclear-envelope vesicles provide the possibility of studying translocation alone. Furthermore, the less complex nature of these vesicles compared with whole nuclei should facilitate investigation of the components involved in the regulation of nuclear transport processes.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aaronson R. P., Blobel G. Isolation of nuclear pore complexes in association with a lamina. Proc Natl Acad Sci U S A. 1975 Mar;72(3):1007–1011. doi: 10.1073/pnas.72.3.1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aaronson R. P., Blobel G. On the attachment of the nuclear pore complex. J Cell Biol. 1974 Sep;62(3):746–754. doi: 10.1083/jcb.62.3.746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agutter P. S. An assessment of some methodological criticisms of studies of RNA efflux from isolated nuclei. Biochem J. 1983 Sep 15;214(3):915–921. doi: 10.1042/bj2140915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agutter P. S., Harris J. R., Stevenson I. Ribonucleic acid stimulation of mammalian liver nuclear-envelope nucleoside triphosphatase. A possible enzymic marker for the nuclear envelope. Biochem J. 1977 Mar 15;162(3):671–679. doi: 10.1042/bj1620671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agutter P. S. Influence of nucleotides, cations and nucleoside triphosphatase inhibitors on the release of ribonucleic acid from isolated rat liver nuclei. Biochem J. 1980 Apr 15;188(1):91–97. doi: 10.1042/bj1880091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agutter P. S., McArdle H. J., McCaldin B. Evidence for involvement of nuclear envelope nucleoside triphosphatase in nucleocytoplasmic translocation of ribonucleoprotein. Nature. 1976 Sep 9;263(5573):165–167. doi: 10.1038/263165a0. [DOI] [PubMed] [Google Scholar]
- BURTON K. A study of the conditions and mechanism of the diphenylamine reaction for the colorimetric estimation of deoxyribonucleic acid. Biochem J. 1956 Feb;62(2):315–323. doi: 10.1042/bj0620315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernd A., Schröder H. C., Zahn R. K., Müller W. E. Modulation of the nuclear-envelope nucleoside triphosphatase by poly(A)-rich mRNA and by microtubule protein. Eur J Biochem. 1982 Dec;129(1):43–49. doi: 10.1111/j.1432-1033.1982.tb07018.x. [DOI] [PubMed] [Google Scholar]
- Blobel G., Potter V. R. Nuclei from rat liver: isolation method that combines purity with high yield. Science. 1966 Dec 30;154(3757):1662–1665. doi: 10.1126/science.154.3757.1662. [DOI] [PubMed] [Google Scholar]
- Bolla R., Roth H. E., Weissbach H., Brot N. Effect of ribosomal proteins on synthesis and assembly of preribosomal particles in isolated rat liver nuclei. J Biol Chem. 1977 Jan 25;252(2):721–725. [PubMed] [Google Scholar]
- Bonner W. M. Protein migration into nuclei. I. Frog oocyte nuclei in vivo accumulate microinjected histones, allow entry to small proteins, and exclude large proteins. J Cell Biol. 1975 Feb;64(2):421–430. doi: 10.1083/jcb.64.2.421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonner W. M. Protein migration into nuclei. II. Frog oocyte nuclei accumulate a class of microinjected oocyte nuclear proteins and exclude a class of microinjected oocyte cytoplasmic proteins. J Cell Biol. 1975 Feb;64(2):431–437. doi: 10.1083/jcb.64.2.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bornens M., Courvalin J. C. Isolation of nuclear envelopes with polyanions. J Cell Biol. 1978 Jan;76(1):191–206. doi: 10.1083/jcb.76.1.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clawson G. A., James J., Woo C. H., Friend D. S., Moody D., Smuckler E. A. Pertinence of nuclear envelope nucleoside triphosphatase activity to ribonucleic acid transport. Biochemistry. 1980 Jun 10;19(12):2748–2756. doi: 10.1021/bi00553a033. [DOI] [PubMed] [Google Scholar]
- Commerford S. L. Iodination of nucleic acids in vitro. Biochemistry. 1971 May 25;10(11):1993–2000. doi: 10.1021/bi00787a005. [DOI] [PubMed] [Google Scholar]
- Cox G. S. Discrimination in the uptake of soluble proteins by isolated nuclei. J Cell Sci. 1982 Dec;58:363–384. doi: 10.1242/jcs.58.1.363. [DOI] [PubMed] [Google Scholar]
- Dabauvalle M. C., Franke W. W. Karyophilic proteins: polypeptides synthesized in vitro accumulate in the nucleus on microinjection into the cytoplasm of amphibian oocytes. Proc Natl Acad Sci U S A. 1982 Sep;79(17):5302–5306. doi: 10.1073/pnas.79.17.5302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davey J., Dimmock N. J., Colman A. Identification of the sequence responsible for the nuclear accumulation of the influenza virus nucleoprotein in Xenopus oocytes. Cell. 1985 Mar;40(3):667–675. doi: 10.1016/0092-8674(85)90215-6. [DOI] [PubMed] [Google Scholar]
- Dingwall C., Sharnick S. V., Laskey R. A. A polypeptide domain that specifies migration of nucleoplasmin into the nucleus. Cell. 1982 Sep;30(2):449–458. doi: 10.1016/0092-8674(82)90242-2. [DOI] [PubMed] [Google Scholar]
- Dwyer N., Blobel G. A modified procedure for the isolation of a pore complex-lamina fraction from rat liver nuclei. J Cell Biol. 1976 Sep;70(3):581–591. doi: 10.1083/jcb.70.3.581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FELDHERR C. M. THE EFFECT OF THE ELECTRON-OPAQUE PORE MATERIAL ON EXCHANGES THROUGH THE NUCLEAR ANNULI. J Cell Biol. 1965 Apr;25:43–53. doi: 10.1083/jcb.25.1.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fais D., Prusov A. N., Polyakov VYu The lack of histone H1 in the peripheral chromatin of rat liver nuclei. Cell Biol Int Rep. 1982 May;6(5):433–441. doi: 10.1016/0309-1651(82)90115-1. [DOI] [PubMed] [Google Scholar]
- Fasold H., Hulla F. W., Ortanderl F., Rack M. Aromatic thioethers of purine nucleotides. Methods Enzymol. 1977;46:289–295. doi: 10.1016/s0076-6879(77)46030-0. [DOI] [PubMed] [Google Scholar]
- Franke W. W., Deumling B., Zentgraf H., Falk H., Rae P. M. Nuclear membranes from mammalian liver. IV. Characterization of membrane-attached DNA. Exp Cell Res. 1973 Oct;81(2):365–392. doi: 10.1016/0014-4827(73)90527-2. [DOI] [PubMed] [Google Scholar]
- Franke W. W., Scheer U., Krohne G., Jarasch E. D. The nuclear envelope and the architecture of the nuclear periphery. J Cell Biol. 1981 Dec;91(3 Pt 2):39s–50s. doi: 10.1083/jcb.91.3.39s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franke W. W. Structure and function of nuclear membranes. Biochem Soc Symp. 1977;(42):125–135. [PubMed] [Google Scholar]
- Gerace L., Blobel G. Nuclear lamina and the structural organization of the nuclear envelope. Cold Spring Harb Symp Quant Biol. 1982;46(Pt 2):967–978. doi: 10.1101/sqb.1982.046.01.090. [DOI] [PubMed] [Google Scholar]
- Gerace L., Blum A., Blobel G. Immunocytochemical localization of the major polypeptides of the nuclear pore complex-lamina fraction. Interphase and mitotic distribution. J Cell Biol. 1978 Nov;79(2 Pt 1):546–566. doi: 10.1083/jcb.79.2.546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurdon J. B. Nuclear transplantation and the control of gene activity in animal development. Proc R Soc Lond B Biol Sci. 1970 Dec 1;176(1044):303–314. doi: 10.1098/rspb.1970.0050. [DOI] [PubMed] [Google Scholar]
- Hall M. N., Hereford L., Herskowitz I. Targeting of E. coli beta-galactosidase to the nucleus in yeast. Cell. 1984 Apr;36(4):1057–1065. doi: 10.1016/0092-8674(84)90055-2. [DOI] [PubMed] [Google Scholar]
- Jacobs H., Birnie G. D. Isolation and purification of rat hepatoma nuclei active in the transport of messenger RNA in vitro. Eur J Biochem. 1982 Jan;121(3):597–607. doi: 10.1111/j.1432-1033.1982.tb05828.x. [DOI] [PubMed] [Google Scholar]
- Kaempfer R. Binding of messenger RNA in initiation of eukaryotic translation. Methods Enzymol. 1979;60:380–392. doi: 10.1016/s0076-6879(79)60036-8. [DOI] [PubMed] [Google Scholar]
- Kalderon D., Roberts B. L., Richardson W. D., Smith A. E. A short amino acid sequence able to specify nuclear location. Cell. 1984 Dec;39(3 Pt 2):499–509. doi: 10.1016/0092-8674(84)90457-4. [DOI] [PubMed] [Google Scholar]
- Kaufmann S. H., Gibson W., Shaper J. H. Characterization of the major polypeptides of the rat liver nuclear envelope. J Biol Chem. 1983 Feb 25;258(4):2710–2719. [PubMed] [Google Scholar]
- Kay R. R., Fraser D., Johnston I. R. A method for the rapid isolation of nuclear membranes from rat liver. Characterisation of the membrane preparation and its associated DNA polymerase. Eur J Biochem. 1972 Oct 17;30(1):145–154. doi: 10.1111/j.1432-1033.1972.tb02081.x. [DOI] [PubMed] [Google Scholar]
- Kirschner R. H., Rusli M., Martin T. E. Characterization of the nuclear envelope, pore complexes, and dense lamina of mouse liver nuclei by high resolution scanning electron microscopy. J Cell Biol. 1977 Jan;72(1):118–132. doi: 10.1083/jcb.72.1.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kletzien R. F., Pariza M. W., Becker J. E., Potter V. R. A method using 3-O-methyl-D-glucose and phloretin for the determination of intracellular water space of cells in monolayer culture. Anal Biochem. 1975 Oct;68(2):537–544. doi: 10.1016/0003-2697(75)90649-1. [DOI] [PubMed] [Google Scholar]
- Kondor-Koch C., Riedel N., Valentin R., Fasold H., Fischer H. Characterization of an ATPase on the inside of rat-liver nuclear envelopes by affinity labeling. Eur J Biochem. 1982 Oct;127(2):285–289. doi: 10.1111/j.1432-1033.1982.tb06868.x. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Maul G. G., Avdalović N. Nuclear envelope proteins from Spisula solidissima germinal vesicles. Exp Cell Res. 1980 Nov;130(1):229–240. doi: 10.1016/0014-4827(80)90059-2. [DOI] [PubMed] [Google Scholar]
- Maul G. G., Baglia F. Localization of a major nuclear envelope protein by differential solubilization. Exp Cell Res. 1983 May;145(2):285–292. doi: 10.1016/0014-4827(83)90007-1. [DOI] [PubMed] [Google Scholar]
- Paine P. L., Feldherr C. M. Nucleocytoplasmic exchange of macromolecules. Exp Cell Res. 1972 Sep;74(1):81–98. doi: 10.1016/0014-4827(72)90483-1. [DOI] [PubMed] [Google Scholar]
- Paine P. L., Moore L. C., Horowitz S. B. Nuclear envelope permeability. Nature. 1975 Mar 13;254(5496):109–114. doi: 10.1038/254109a0. [DOI] [PubMed] [Google Scholar]
- Paine P. L., Pearson T. W., Tluczek L. J., Horowitz S. B. Nuclear sodium and potassium. Nature. 1981 May 21;291(5812):258–259. doi: 10.1038/291258a0. [DOI] [PubMed] [Google Scholar]
- Palayoor T., Schumm D. E., Webb T. E. Transport of functional messenger RNA from liver nuclei in a reconstituted cell-free system. Biochim Biophys Acta. 1981 Jul 27;654(2):201–210. doi: 10.1016/0005-2787(81)90173-8. [DOI] [PubMed] [Google Scholar]
- Palmiter R. D. Magnesium precipitation of ribonucleoprotein complexes. Expedient techniques for the isolation of undergraded polysomes and messenger ribonucleic acid. Biochemistry. 1974 Aug 13;13(17):3606–3615. doi: 10.1021/bi00714a032. [DOI] [PubMed] [Google Scholar]
- Rechsteiner M., Kuehl L. Microinjection of the nonhistone chromosomal protein HMG1 into bovine fibroblasts and HeLa cells. Cell. 1979 Apr;16(4):901–908. doi: 10.1016/0092-8674(79)90105-3. [DOI] [PubMed] [Google Scholar]
- Richardson J. C., Maddy A. H. The polypeptides of rat liver nuclear envelope. I. Examination by nuclear pore complex polypeptides by solid-state lactoperoxidase labelling. J Cell Sci. 1980 Jun;43:253–267. doi: 10.1242/jcs.43.1.253. [DOI] [PubMed] [Google Scholar]
- Richardson W. D., Roberts B. L., Smith A. E. Nuclear location signals in polyoma virus large-T. Cell. 1986 Jan 17;44(1):77–85. doi: 10.1016/0092-8674(86)90486-1. [DOI] [PubMed] [Google Scholar]
- Riedel N., Fasold H. Nuclear-envelope vesicles as a model system to study nucleocytoplasmic transport. Specific uptake of nuclear proteins. Biochem J. 1987 Jan 1;241(1):213–219. doi: 10.1042/bj2410213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schatten G., Thoman M. Nuclear surface complex as observed with the high resolution scanning electron microscope. Visualization of the membrane surfaces of the neclear envelope and the nuclear cortex from Xenopus laevis oocytes. J Cell Biol. 1978 May;77(2):517–535. doi: 10.1083/jcb.77.2.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schröder H. C., Rottmann M., Bachmann M., Müller W. E. Purification and characterization of the major nucleoside triphosphatase from rat liver nuclear envelopes. J Biol Chem. 1986 Jan 15;261(2):663–668. [PubMed] [Google Scholar]
- Schumm D. E., Morris H. P., Webb T. E. Cytosol-modulated transport of messenger RNA from isolated nuclei. Cancer Res. 1973 Aug;33(8):1821–1828. [PubMed] [Google Scholar]
- Sikstrom R., Lanoix J., Bergeron J. J. An enzymic analysis of a nuclear envelope fraction. Biochim Biophys Acta. 1976 Sep 21;448(1):88–102. doi: 10.1016/0005-2736(76)90078-x. [DOI] [PubMed] [Google Scholar]
- Silver P. A., Keegan L. P., Ptashne M. Amino terminus of the yeast GAL4 gene product is sufficient for nuclear localization. Proc Natl Acad Sci U S A. 1984 Oct;81(19):5951–5955. doi: 10.1073/pnas.81.19.5951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuart S. E., Clawson G. A., Rottman F. M., Patterson R. J. RNA transport in isolated myeloma nuclei. Transport from membrane-denuded nuclei. J Cell Biol. 1977 Jan;72(1):57–66. doi: 10.1083/jcb.72.1.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaizumi M., Uchida T., Okada Y., Furusawa M., Mitsui H. Rapid transfer of non-histone chromosomal proteins to the nucleus of living cells. Nature. 1978 Jun 29;273(5665):782–784. doi: 10.1038/273782a0. [DOI] [PubMed] [Google Scholar]
- Zbarsky I. B. An enzyme profile of the nuclear envelope. Int Rev Cytol. 1978;54:295–360. doi: 10.1016/s0074-7696(08)60172-2. [DOI] [PubMed] [Google Scholar]