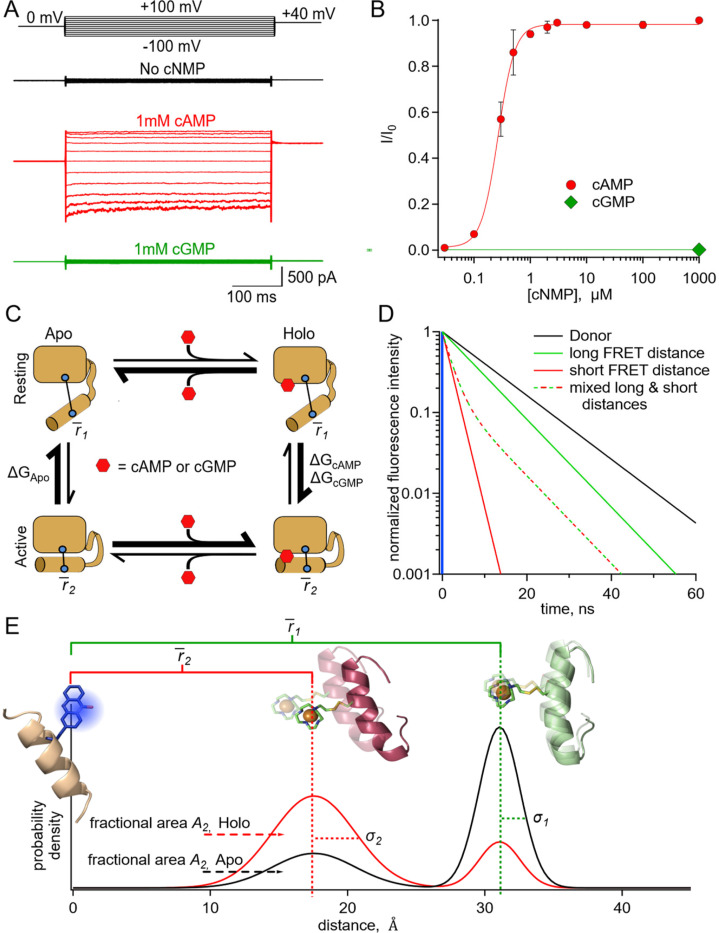
Fig. 1.
SthK as a model protein for characterizing the allosteric regulation in CNBD channels. (A) Representative macroscopic A208V-cfSthK currents in inside-out patches from bacterial spheroplasts in response to voltage steps shown at the top, in the absence of cyclic nucleotide (top black trace), in saturating 1 mM cAMP (middle red trace), and in saturating 1 mM cGMP (bottom green trace). (B) Dose-response relation of A208V-cfSthK to cAMP at +80 mV (red circles, n=6), fit with the Hill Equation (in red, : 0.27 ± 0.01 μM, slope: 2.9 ± 0.2, ± SD). Fractional activation by 1 mM cGMP was 0.002 ± 0.003 (± SD) (green diamond, n=6). (C) State diagram showing four states (either apo or holo, and either resting or active) of the CNBD and the associated ’s for the transitions between states. (D) Theoretical fluorescence lifetime decays of a donor fluorophore in the time-domain showing basis of time-resolved tmFRET. A single exponential donor and mixtures of two single tmFRET distances (short and long) are shown. (E) Theoretical distance distributions showing two states with average distances, , heterogeneity within each conformational state as standard deviation, , and heterogeneity between conformational states as fractional area for apo (black) and holo (red) conditions.