Abstract
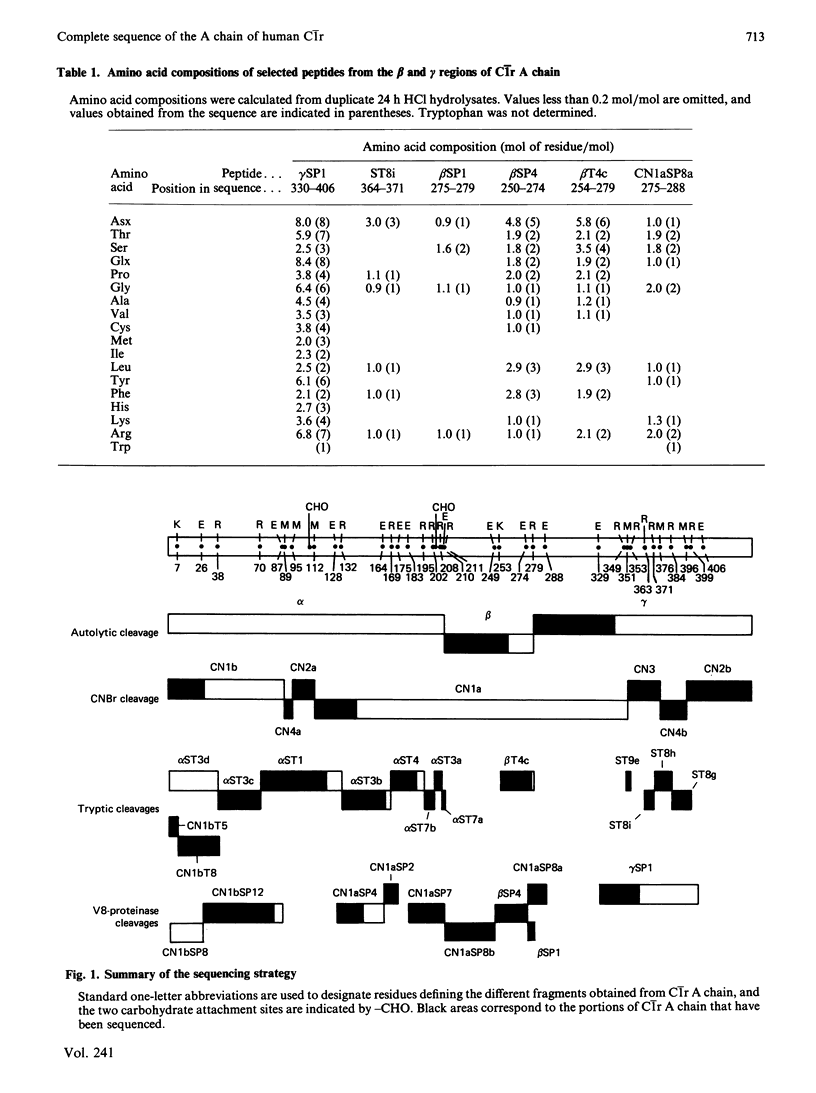
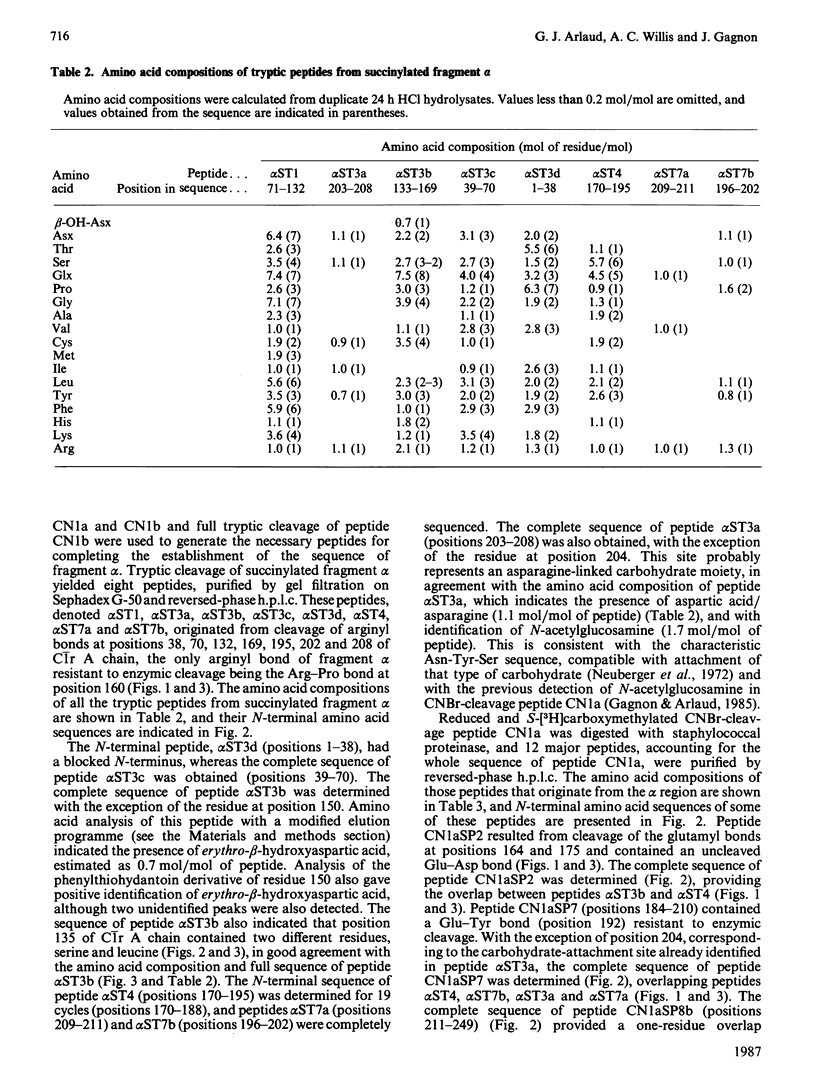
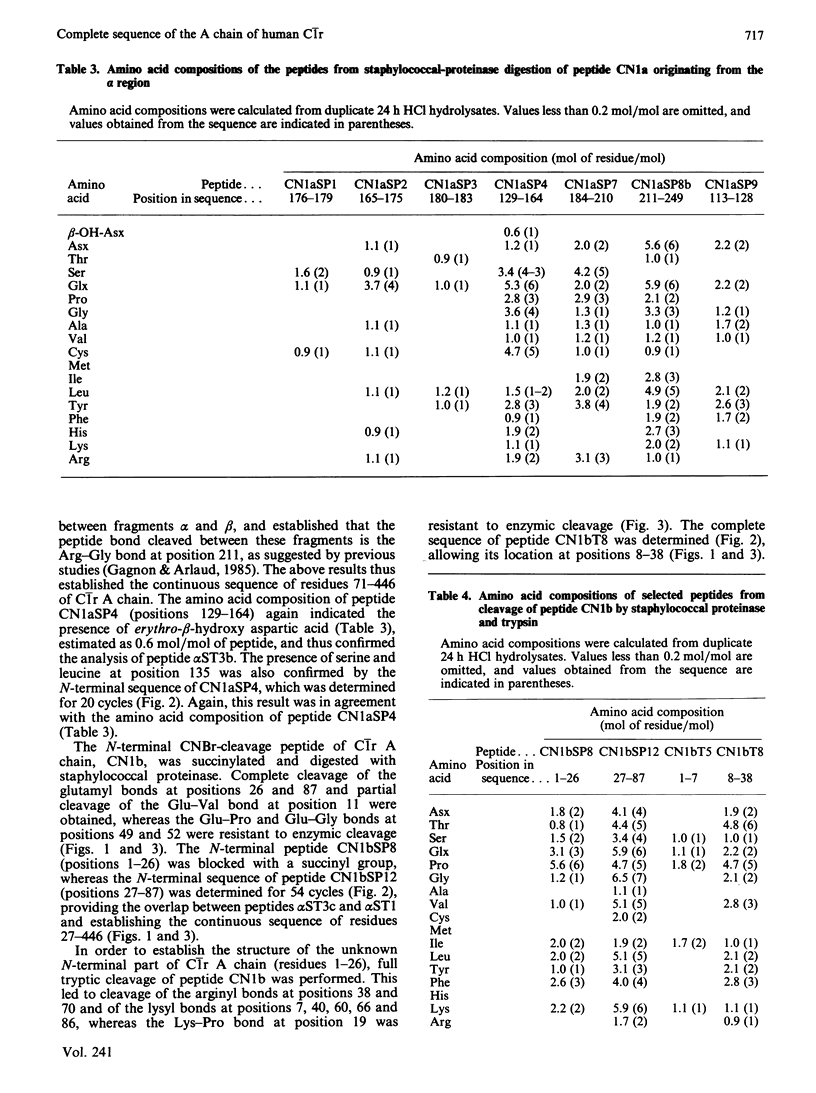
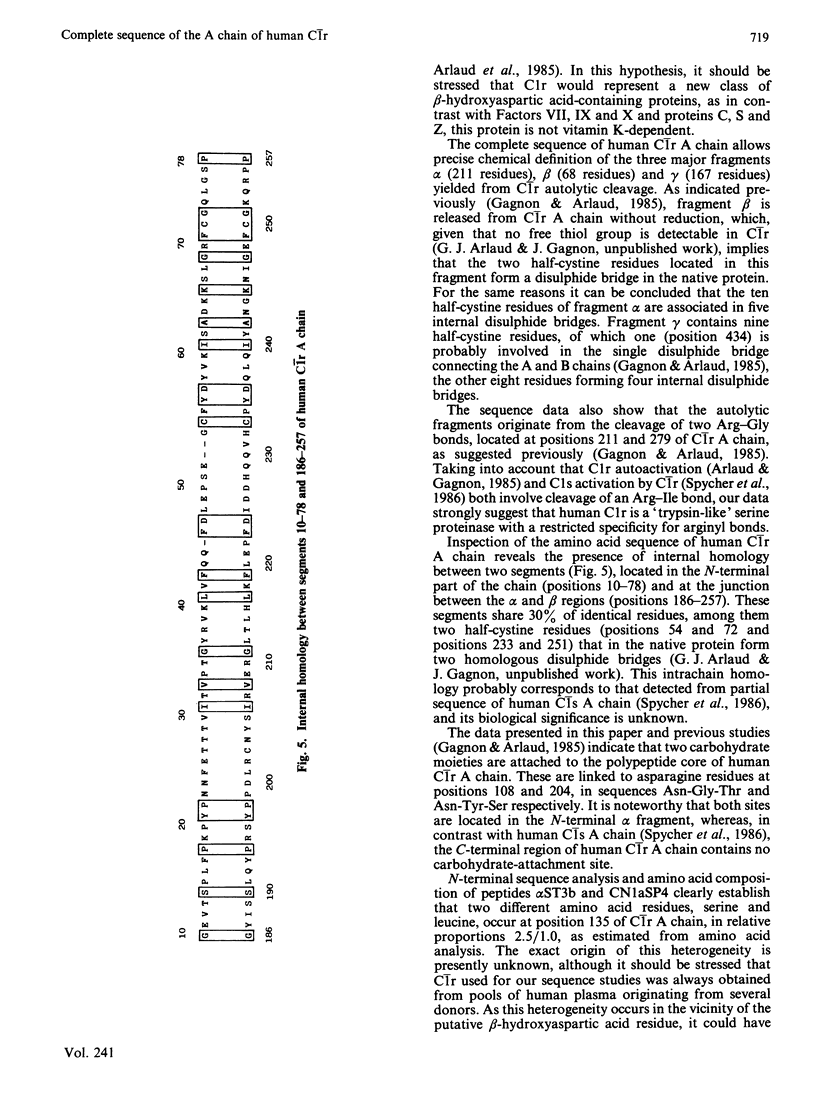
The amino acid sequence of human C1r A chain was determined, from sequence analysis performed on fragments obtained from C1r autolytic cleavage, cleavage of methionyl bonds, tryptic cleavages at arginine and lysine residues, and cleavages by staphylococcal proteinase. The polypeptide chain has an N-terminal serine residue and contains 446 amino acid residues (Mr 51,200). The sequence data allow chemical characterization of fragments alpha (positions 1-211), beta (positions 212-279) and gamma (positions 280-446) yielded from C1r autolytic cleavage, and identification of the two major cleavage sites generating these fragments. Position 150 of C1r A chain is occupied by a modified amino acid residue that, upon acid hydrolysis, yields erythro-beta-hydroxyaspartic acid, and that is located in a sequence homologous to the beta-hydroxyaspartic acid-containing regions of Factor IX, Factor X, protein C and protein Z. Sequence comparison reveals internal homology between two segments (positions 10-78 and 186-257). Two carbohydrate moieties are attached to the polypeptide chain, both via asparagine residues at positions 108 and 204. Combined with the previously determined sequence of C1r B chain [Arlaud & Gagnon (1983) Biochemistry 22, 1758-1764], these data give the complete sequence of human C1r.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anson D. S., Choo K. H., Rees D. J., Giannelli F., Gould K., Huddleston J. A., Brownlee G. G. The gene structure of human anti-haemophilic factor IX. EMBO J. 1984 May;3(5):1053–1060. doi: 10.1002/j.1460-2075.1984.tb01926.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arlaud G. J., Colomb M. G., Villiers C. L. C1r serine proteinase of human complement: a case of intramolecular autolytic activation. Biosci Rep. 1985 Oct-Nov;5(10-11):831–837. doi: 10.1007/BF01119894. [DOI] [PubMed] [Google Scholar]
- Arlaud G. J., Gagnon J. Complete amino acid sequence of the catalytic chain of human complement subcomponent C1-r. Biochemistry. 1983 Apr 12;22(8):1758–1764. doi: 10.1021/bi00277a003. [DOI] [PubMed] [Google Scholar]
- Arlaud G. J., Gagnon J., Porter R. R. The catalytic chain of human complement subcomponent C1r. Purification and N-terminal amino acid sequences of the major cyanogen bromide-cleavage fragments. Biochem J. 1982 Jan 1;201(1):49–59. doi: 10.1042/bj2010049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arlaud G. J., Sim R. B., Duplaa A. M., Colomb M. G. Differential elution of Clq, Clr and Cls from human Cl bound to immune aggregates. Use in the rapid purification of Cl subcomponents. Mol Immunol. 1979 Jul;16(7):445–450. doi: 10.1016/0161-5890(79)90069-5. [DOI] [PubMed] [Google Scholar]
- Arlaud G. J., Villiers C. L., Chesne S., Colomb M. G. Purified proenzyme C1r. Some characteristics of its activation and subsequent proteolytic cleavage. Biochim Biophys Acta. 1980 Nov 6;616(1):116–129. doi: 10.1016/0005-2744(80)90269-7. [DOI] [PubMed] [Google Scholar]
- Christie D. L., Gagnon J. Isolation, characterization and N-terminal sequences of the CNBr-cleavage peptides from human complement Factor B. Localization of a free thiol group and a sequence defining the site cleaved by factor D. Biochem J. 1982 Mar 1;201(3):555–567. doi: 10.1042/bj2010555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colomb M. G., Arlaud G. J., Villiers C. L. Activation of C1. Philos Trans R Soc Lond B Biol Sci. 1984 Sep 6;306(1129):283–292. doi: 10.1098/rstb.1984.0089. [DOI] [PubMed] [Google Scholar]
- Cooper N. R. The classical complement pathway: activation and regulation of the first complement component. Adv Immunol. 1985;37:151–216. doi: 10.1016/s0065-2776(08)60340-5. [DOI] [PubMed] [Google Scholar]
- Drakenberg T., Fernlund P., Roepstorff P., Stenflo J. beta-Hydroxyaspartic acid in vitamin K-dependent protein C. Proc Natl Acad Sci U S A. 1983 Apr;80(7):1802–1806. doi: 10.1073/pnas.80.7.1802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernlund P., Stenflo J. Beta-hydroxyaspartic acid in vitamin K-dependent proteins. J Biol Chem. 1983 Oct 25;258(20):12509–12512. [PubMed] [Google Scholar]
- Foster D. C., Yoshitake S., Davie E. W. The nucleotide sequence of the gene for human protein C. Proc Natl Acad Sci U S A. 1985 Jul;82(14):4673–4677. doi: 10.1073/pnas.82.14.4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gagnon J., Arlaud G. J. Primary structure of the A chain of human complement-classical-pathway enzyme C1r. N-terminal sequences and alignment of autolytic fragments and CNBr-cleavage peptides. Biochem J. 1985 Jan 1;225(1):135–142. doi: 10.1042/bj2250135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunkapiller M. W., Hood L. E. Direct microsequence analysis of polypeptides using an improved sequenator, a nonprotein carrier (polybrene), and high pressure liquid chromatography. Biochemistry. 1978 May 30;17(11):2124–2133. doi: 10.1021/bi00604a016. [DOI] [PubMed] [Google Scholar]
- Højrup P., Jensen M. S., Petersen T. E. Amino acid sequence of bovine protein Z: a vitamin K-dependent serine protease homolog. FEBS Lett. 1985 May 20;184(2):333–338. doi: 10.1016/0014-5793(85)80633-5. [DOI] [PubMed] [Google Scholar]
- KORNGUTH M. L., SALLACH H. J. beta-Hydroxyaspartic acid: synthesis and separation of its diastereoisomers. Arch Biochem Biophys. 1960 Nov;91:39–42. doi: 10.1016/0003-9861(60)90451-3. [DOI] [PubMed] [Google Scholar]
- Long G. L., Belagaje R. M., MacGillivray R. T. Cloning and sequencing of liver cDNA coding for bovine protein C. Proc Natl Acad Sci U S A. 1984 Sep;81(18):5653–5656. doi: 10.1073/pnas.81.18.5653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMullen B. A., Fujikawa K., Kisiel W., Sasagawa T., Howald W. N., Kwa E. Y., Weinstein B. Complete amino acid sequence of the light chain of human blood coagulation factor X: evidence for identification of residue 63 as beta-hydroxyaspartic acid. Biochemistry. 1983 Jun 7;22(12):2875–2884. doi: 10.1021/bi00281a016. [DOI] [PubMed] [Google Scholar]
- McMullen B. A., Fujikawa K., Kisiel W. The occurrence of beta-hydroxyaspartic acid in the vitamin K-dependent blood coagulation zymogens. Biochem Biophys Res Commun. 1983 Aug 30;115(1):8–14. doi: 10.1016/0006-291x(83)90961-0. [DOI] [PubMed] [Google Scholar]
- Morita T., Isaacs B. S., Esmon C. T., Johnson A. E. Derivatives of blood coagulation factor IX contain a high affinity Ca2+-binding site that lacks gamma-carboxyglutamic acid. J Biol Chem. 1984 May 10;259(9):5698–5704. [PubMed] [Google Scholar]
- Morita T., Isaacs B. S., Esmon C. T., Johnson A. E. Derivatives of blood coagulation factor IX contain a high affinity Ca2+-binding site that lacks gamma-carboxyglutamic acid. J Biol Chem. 1984 May 10;259(9):5698–5704. [PubMed] [Google Scholar]
- Reid K. B., Porter R. R. The proteolytic activation systems of complement. Annu Rev Biochem. 1981;50:433–464. doi: 10.1146/annurev.bi.50.070181.002245. [DOI] [PubMed] [Google Scholar]
- Sim R. B., Porter R. R., Reid K. B., Gigli I. The structure and enzymic activities of the C1r and C1s subcomponents of C1, the first component of human serum complement. Biochem J. 1977 May 1;163(2):219–227. doi: 10.1042/bj1630219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sim R. B. The human complement system serine proteases C1r and C1s and their proenzymes. Methods Enzymol. 1981;80(Pt 100):26–42. doi: 10.1016/s0076-6879(81)80006-7. [DOI] [PubMed] [Google Scholar]
- Spycher S. E., Nick H., Rickli E. E. Human complement component C1s. Partial sequence determination of the heavy chain and identification of the peptide bond cleaved during activation. Eur J Biochem. 1986 Apr 1;156(1):49–57. doi: 10.1111/j.1432-1033.1986.tb09546.x. [DOI] [PubMed] [Google Scholar]
- Sugo T., Björk I., Holmgren A., Stenflo J. Calcium-binding properties of bovine factor X lacking the gamma-carboxyglutamic acid-containing region. J Biol Chem. 1984 May 10;259(9):5705–5710. [PubMed] [Google Scholar]
- Sugo T., Fernlund P., Stenflo J. erythro-beta-Hydroxyaspartic acid in bovine factor IX and factor X. FEBS Lett. 1984 Jan 2;165(1):102–106. doi: 10.1016/0014-5793(84)80023-x. [DOI] [PubMed] [Google Scholar]
- Villiers C. L., Arlaud G. J., Colomb M. G. Domain structure and associated functions of subcomponents C1r and C1s of the first component of human complement. Proc Natl Acad Sci U S A. 1985 Jul;82(13):4477–4481. doi: 10.1073/pnas.82.13.4477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villiers C. L., Arlaud G. J., Painter R. H., Colomb M. G. Calcium binding properties of the C1 subcomponents C1q, C1r and C1s. FEBS Lett. 1980 Aug 11;117(1):289–294. doi: 10.1016/0014-5793(80)80964-1. [DOI] [PubMed] [Google Scholar]
- Villiers C. L., Arlaud G. J., Painter R. H., Colomb M. G. Calcium binding properties of the C1 subcomponents C1q, C1r and C1s. FEBS Lett. 1980 Aug 11;117(1):289–294. doi: 10.1016/0014-5793(80)80964-1. [DOI] [PubMed] [Google Scholar]


