Abstract
Purpose
This study aimed to identify environmental risk factors associated with asthmatic fixed airflow obstruction (FAO) and assess the relationship between small airway abnormalities defined by forced expiratory flow at 25–75% (FEF25-75%) and FAO.
Patients and Methods
We analyzed data from 312 han Chinese patients with stable asthma on standard treatment. Low FEF25-75% was defined as post-bronchodilator FEF25-75% z-score <−0.8435, and FAO as post-bronchodilator FEV1/FVC z-score <−1.645. Exposure levels were retrospectively analyzed in relation to FAO risk in asthmatics. Asthmatics were grouped by low FEF25-75% and FAO, and lung function, environmental exposure, daily symptoms, and exacerbations in the previous year were compared cross-sectionally across groups.
Results
In retrospective analyses, pack-years of smoking in male patients (adjusted odd ratio [95% confidence interval] 1.05 [1.03–1.07], P<0.001), biomass exposure for >20 years (2.65 [1.13–6.43], P=0.027), occupational exposure for >10 years (2.01 [1.06–3.86], P=0.035) and occupational exposure for >20 years (2.67 [1.24–5.91], P=0.013) were associated with asthmatic FAO. In cross-sectional analyses, compared with the normal FEF25-75%/ asthmatics without FAO (NON-FAO) group, the low FEF25-75%/ asthmatics with FAO (FAO) group had lower FEV1 z-scores and FEV1/FVC z-scores, more pack-years and years of biomass and occupational exposure, higher Asthma Control Questionnaire-5 and Chronic Obstructive Pulmonary Disease Assessment Test scores, and more frequent exacerbations. The low FEF25-75%/NON-FAO group showed the same trend, but to a lesser extent.
Conclusion
Chronic airway inflammation is not the only driver of asthmatic FAO, and management and treatment targeting environmental risk factors (smoking and biomass and occupational exposures) may slow FAO progression in asthmatics. The FEF25-75% determined by the z-score is a reliable marker of small airway abnormalities, and patients with low FEF25-75% are at greater risk for FAO, requiring more frequent follow-up.
Keywords: asthma, fixed airflow obstruction, environmental exposures, forced expiratory flow at 25-75%
Introduction
Adults with a long history of asthma may have coexisting fixed airflow obstruction (FAO), which is associated with more respiratory symptoms, a poorer quality of life, and a greater risk of death.1 The long-standing paradigm that airway remodeling driven by persistent airway inflammation is the sole driver of FAO in asthma is controversial, and other pathophysiological mechanisms may act synergistically with inflammation to cause FAO.2 In the past, many forms of environmental exposure were considered risk factors for FAO only in chronic obstructive pulmonary disease (COPD), but in recent years, various environmental exposures have also been found to be associated with FAO in asthma. For example, tobacco smoke can exacerbate airway remodeling in asthmatics by affecting airway inflammation or airway damage repair mechanisms.3 In addition, a real-world study found that severe acute dust and smoke exposure were risk factors for COPD or FAO in workers with asthma.4 However, whether chronic and sustained environmental exposure affects FAO development in asthmatics remains unknown.
Asthmatic FAO generally refers to the failure to normalize spirometry results within minutes after inhalation of a rapid-acting bronchodilator or over days or weeks after the initiation of an effective controller treatment, such as an inhaled corticosteroid (ICS).1,2,5 The spirometry criterion usually chosen for the diagnosis of FAO is post-bronchodilator FEV1/FVC < 0.7 because this criterion is simple to measure and is independent of reference values.6 An alternative diagnosis is based on the z-score to define airflow obstruction (lower limit of normal, LLN) and stratify its severity. The z-score, which represents the number of standard deviations (SDs) of the difference between the measured and mean predicted values, is statistically and scientifically more reliable, reduces the misdiagnosis of pathological airflow obstruction, and allows for the earlier detection of milder forms of the disease.5–8 In addition, the determination of lung function abnormalities diagnosed on the basis of z-scores does not add to the burden of a busy clinical workload. Z-scores are calculated from ethnically diverse, all-age prediction equations published by the Global Lung Function Initiative. Similar to abnormalities defined by percentage reference, the interpretation of lung function abnormalities defined by z-scores is based on comparison of the subject’s actual measurements with an abnormal reference z-value.5,7,8
Undeniably, the diagnostic threshold for FAO can only be exceeded after the development of severe irreversible pathological changes in the lungs, which virtually eliminates the possibility of a cure because such changes are almost certainly permanent. Asthmatics with comorbid FAO experience the greatest impairment in health status and consume the greatest proportion of healthcare resources. Detection of airflow obstruction by more sensitive pulmonary function tests or detection of pathological changes by imaging can help identify asthmatics with early pathological changes, leading to a greater likelihood of understanding the mechanistic pathways by which asthmatic FAO develops and thus to the development of effective therapies to halt and reverse its progression. Small airways, usually defined as airways with an internal diameter <2 mm and without cartilage, are the main sites of airflow obstruction in asthma and COPD.9 Previous studies on the relationship between small airway damage and FAO development have focused almost exclusively on COPD patients, confirming that small airway damage is a key factor contributing to airflow obstruction in early COPD.10,11 However, pathological abnormalities of the small airways are equally common in asthmatics with either T2 or non-T2 endotypes,12 which suggests that indicators of small airway damage in asthmatics may also have significant predictive value in the early identification of the development of FAO in asthmatics.
FEF25-75% is currently the most commonly cited parameter to identify small airway obstruction and can reflect early small airway damage more sensitively than traditional spirometry measures. Although the parameter is highly variable, the use of z-scores reduces this variability by eliminating the bias associated with age, sex, and height.7,13,14 The optimal z-score cutoff for predicting the development of FAO using FEF25-75% is −0.8435 (20th percentile of the lower limit of normal for FEF25-75%), which was derived from an observational cohort study that revealed that in the presence of normal lung function (defined as FEV1/FVC >0.7, FVC >80% of the predicted value, and FEV1 >80% of the predicted value), patients with FEF25-75% z-scores <−0.8435 had a significantly greater risk of developing FAO at 10 years than patients with z-scores above this cutoff.11 This finding was supported by a subsequent cross-sectional study in a smoking population, which revealed that the use of FEF25-75% z-scores could identify early pathological features of airflow obstruction.10 In asthmatics, this cutoff may be equally valuable for the early identification of asthmatic FAO.
We hypothesized that asthmatic FAO is not only a result of persistent inflammation from asthma itself but that environmental exposure also contributes. We also hypothesized that small airway damage is associated with the development of asthmatic FAO and that an FEF25-75%, as measured by the z-score, can be used to detect early pathological features of asthmatic FAO. Accordingly, we set the following objectives:
To investigate the relationship between environmental exposure and asthmatic FAO.
To investigate the prevalence of low FEF25-75% (measured by z-score) in asthmatics with and without FAO.
To evaluate the stability of the FEF25-75% z-score and its correlation with other spirometric parameters.
To categorize asthmatics into normal FEF25-75%/NON-FAO, low FEF25-75%/NON-FAO, and low FEF25-75%/FAO groups based on the presence or absence of low FEF25-75% and FAO, and to compare the differences between the three groups in terms of lung function, daily symptoms, environmental exposure, and exacerbations.
As an additional part of this study, we validated the applicability of the Chronic Obstructive Pulmonary Disease Assessment Test (CAT) score for symptom assessment in asthmatics with and without FAO.
Materials and Methods
Study Design and Participants
This study aimed to identify potential environmental risk factors associated with asthmatic FAO and determine the role of FEF25-75% in the early identification of the development of asthmatic FAO. Patients with asthma who visited Henan Provincial People’s Hospital between December 1, 2022, and January 31, 2024, and underwent routine pulmonary function testing and reversibility testing were included. The study population included patients with pure asthma and asthmatic patients with FAO (defined as patients whose asthma preceded FAO development). The present study was conducted in accordance with the Declaration of Helsinki and approved by the Medical Ethics Committee of Henan Provincial People’s Hospital (NO.202105602), and all participants provided informed consent. Distinguishing asthmatics with FAO from patients with COPD is problematic, particularly in those with asthma-COPD overlap (ACO), who have a long history of asthma, a history of modest smoking, and FAO.15 ACO does not refer to a single disease or syndrome but rather to a complex interplay and combination of different pathophysiological mechanisms. A single definition may influence the choice of optimal medication in clinical practice.15,16 Further subtyping of ACOs based on host factors may allow these patients to benefit from effective pharmacological measures based on asthma and/or COPD diagnosis.15 In this study, greater emphasis was placed on the development of FAO in the context of asthma; therefore, the definition of “asthma with FAO” in this study depended largely on the sequence in which the two conditions were diagnosed.
Eligibility Criteria
All asthmatics were screened for inclusion in this study. Eligible patients with those stable asthma who were already receiving standard asthma medications and had a long history of asthma that preceded the development of FAO.
The inclusion criteria for this study were as follows:
Age between 40 and 80 years.
Had a diagnosis of asthma confirmed by positive pulmonary function provocation test or positive bronchodilator reversibility test.
Had symptoms of wheezing, shortness of breath, chest tightness, and/or cough that varied over time and in intensity in the early stages of the disease with onset before the age of 40 years.
Participants were currently clinically stable, with at least 30 days since their last exacerbation.
Participants with the following conditions were excluded:
Those who had used only bronchodilators for treatment, never used an asthma controller containing an ICS or leukotriene receptor antagonist, or discontinued or failed to adhere to the use of an asthma controller without medical advice.
Those with a history of chronic lung diseases other than asthma, such as tuberculosis, bronchiectasis, and interstitial fibrosis. Patients with current chronic bronchitis (CB) or emphysema diagnosed by imaging were not excluded. Studies have shown that current asthma is strongly and independently associated with CB in middle-aged individuals and that this condition may be even more influential than personal smoking.17 Another study confirmed that asthmatics with FAO had significant parenchymal destruction, regardless of smoking status and asthma severity.18
Those with medical conditions that may affect their symptoms or lung function measurements, such as malignancy, severe cardiovascular disease, and psychiatric disorders.
Definitions and Measurements
After the eligibility assessment, the patients’ sociodemographic data, history of environmental exposure, medical history, Asthma Control Questionnaire-5 (ACQ-5) scores, CAT scores, and lung function test results were collected.
Environmental exposures included smoking, biomass exposure, and occupational exposure. Assessment of smoking history included current smoking status and the amount smoked. Smokers were identified by a history of smoking at least 100 cigarettes, whereas ex-smokers were defined as those who had quit smoking ≥6 months before the study. Passive smoking was assessed by asking whether the patient had lived with a smoker for >6 months. Biomass exposure was defined as exposure to indoor combustion sources that primarily used (1) coal or charcoal or (2) wood, agricultural crop residues, or dung for cooking or heating for ≥2 hours per day for a cumulative lifetime exposure of ≥6 months. Occupational exposure was defined as prolonged (≥6 months) exposure to organic or inorganic dust, chemicals, and gases. The severity of environmental exposure other than smoking was expressed as years of cumulative exposure. The cutoff values for environmental exposure included 6 months, 10 years, and 20 years, which were used to investigate the relationship between the level of environmental exposure and FAO.
Medical history included the age of asthma onset, duration of asthma, number of exacerbations in the past year, and comorbidities. Questions to assess age of asthma onset and duration included “When did you first experience symptoms such as episodic wheezing and shortness of breath?” (<25 years old or ≥25 years old) and “How many years have passed since you first experienced these symptoms?” (≤25 years, 26–50 years, >50 years). The age of 25 years was chosen as the cutoff between early- and late-onset asthma based on the continuous improvement in lung function from birth to early adulthood, which typically peaks between the ages of 20 and 25 years. Asthma occurring before this age may result in pulmonary hypoplasia.19 The choice of the asthma duration cutoff was determined by the current age of the asthma participants and the age of asthma onset (the probable duration of asthma for the participants in this study was 0–80 years). Asthma severity is reflected in daily symptoms and frequency of exacerbations, as these are the main characteristics that influence the difficulty of treatment and severity classification of asthma. Exacerbations were defined as symptoms that exceeded the usual status that required systemic steroids and/or increased doses of ICSs to achieve relief. Severe exacerbations were defined as symptoms that exceeded the usual status and were not relieved by systemic steroids, thus requiring emergency care or hospitalization. The collection of comorbidities included CB and allergic diseases, including allergic rhinitis, allergic conjunctivitis, and urticaria, occurring before the age of 40 years. CB was defined as chronic cough and sputum production for ≥3 months per year for 2 consecutive years.6 Allergic comorbidities in asthmatics suggest type 2 inflammation, whereas patients with non-type 2 inflammation do not have an allergic component in their bodies, and the immune response is mainly triggered by pollutants and inhalant irritants.20
The ACQ-5 and CAT were used to assess the daily symptoms in the asthmatics in this study. The ACQ-5 is a shortened version of the ACQ-7, does not affect the assessment of asthma control, and is not affected by the use of formoterol (a long-acting, rapid-acting β-agonist) in combination with an ICS as a reliever, as recommended by the Global Initiative for Asthma (GINA).1,21 CAT is typically used to assess COPD rather than asthma. However, the CAT score has been shown to be strongly correlated with the ACQ score, especially when applied to patients with asthma and ACO.22 Based on the content of the questionnaire, the CAT provides a more comprehensive assessment of the functional impact of patients’ respiratory symptoms on health and daily life than the ACQ-5.23 The present study aimed to validate the correlation between CAT and ACQ-5 scores and the applicability of CAT in asthmatics with and without FAO.
All lung function measurements were obtained after bronchodilator administration. FEF25-75% z-score, FEF25-75%/FVC ratio, FEV1 z-score, FVC z-score, and FEV1/FVC z-score were recorded. FAO was defined by post-bronchodilator FEV1/FVC below the LLN (z-score <−1.645), with severity categorized based on FEV1 z-score.7 Low FEF25-75% was defined as a post-bronchodilator FEF25-75% z-score <−0.8435.15,20 Calculation of z-scores was based on reference equations established by the Global Lung Function Initiative in 2012 for the Northeast Asian population.8
Statistical Analysis
All statistical analyses were performed using R 4.2.2 and SPSS 26. A P value < 0.05 was considered to indicate statistical significance. The normality of data distribution was assessed using histograms and normal QQ plots. Descriptive statistics for categorical variables are presented as counts and percentages, continuous variables that followed a normal distribution are presented as mean and SD, and those that did not follow a normal distribution are presented as median and interquartile range (IQR).
We performed three independent comparisons: (1) Asthmatics without FAO (NON-FAO) versus asthmatics with FAO (FAO). Depending on the type of variable, we used the t-test, Fisher’s exact test, and Kruskal–Wallis test to analyze known determinants (including age, sex, pack-years, age at asthma onset, and duration of asthma) and potential determinants (allergic comorbidities) of asthmatic FAO development. Variables with a P value < 0.1 in the univariate analysis were then selected as covariates for the multivariate analysis. Environmental risk factors for the development of asthmatic FAO were evaluated using a multivariate logistic regression model, with the results presented as odds ratios (ORs) and 95% confidence intervals (CIs). The adjusted covariates are shown in Table 1. (2) Correlations between various lung function parameters, ACQ-5, and CAT were evaluated using Spearman’s rank correlation analysis and expressed as a matrix of correlation coefficients. (3) The normal FEF25-75%/NON-FAO group versus low FEF25-75%/NON-FAO group versus low FEF25-75%/FAO group. The Kruskal–Wallis test and Fisher’s exact test were used for comparisons among the three groups, depending on the type of variable. For multiple testing, the P value was corrected using the Bonferroni method, and the adjusted P value significance level was set at P<0.05. In addition, the Jonckheere–Terpstra trend test was used when the Kruskal–Wallis test indicated a significant association, and the Mantel–Haenszel trend test was used when Fisher’s exact test indicated a significant association. The order of the severity of airflow obstruction among the three groups was a prerequisite for the trend test.
Table 1.
Covariate Analysis Related to the Development of Asthmatic FAO
| Variable | Total | Non-FAO | FAO | P |
|---|---|---|---|---|
| n=312 | n=170 | n=142 | ||
| Age (years) | 59.8 (9.80) | 60.0 (9.98) | 59.6 (9.61) | 0.729 |
| Sex | 0.017 | |||
| Female | 156 (50.0) | 96 (56.5) | 60 (42.3) | |
| Male | 156 (50.0) | 74 (43.5) | 82 (57.7) | |
| Pack-yearsa | 10.0 [0.00;30.0] | 0.00 [0.00;19.0] | 20.0 [0.00;39.2] | <0.001 |
| Age of asthma onset | <0.001 | |||
| <25 years old | 49 (15.7) | 11 (6.47) | 38 (26.8) | |
| ≥25 years old | 263 (84.3) | 159 (93.5) | 104 (73.2) | |
| Duration of asthma (years) | 0.008 | |||
| 0~25 | 161 (51.6) | 98 (57.6) | 63 (44.4) | |
| 26~50 | 132 (42.3) | 67 (39.4) | 65 (45.8) | |
| >50 | 19 (6.09) | 5 (2.94) | 14 (9.86) | |
| Allergic comorbiditiesb | 104 (33.3) | 49 (28.8) | 55 (38.7) | 0.071 |
Notes: Categorical variables were presented as counts (percentages). Continuous variables with a normal distribution were presented as mean (SD), otherwise as median (IQR). aOnly in male participants, bAllergic comorbidities included one or more allergic diseases that onset before the age of 40, such as allergic rhinitis, allergic conjunctivitis, and urticaria.
Results
Study Subjects
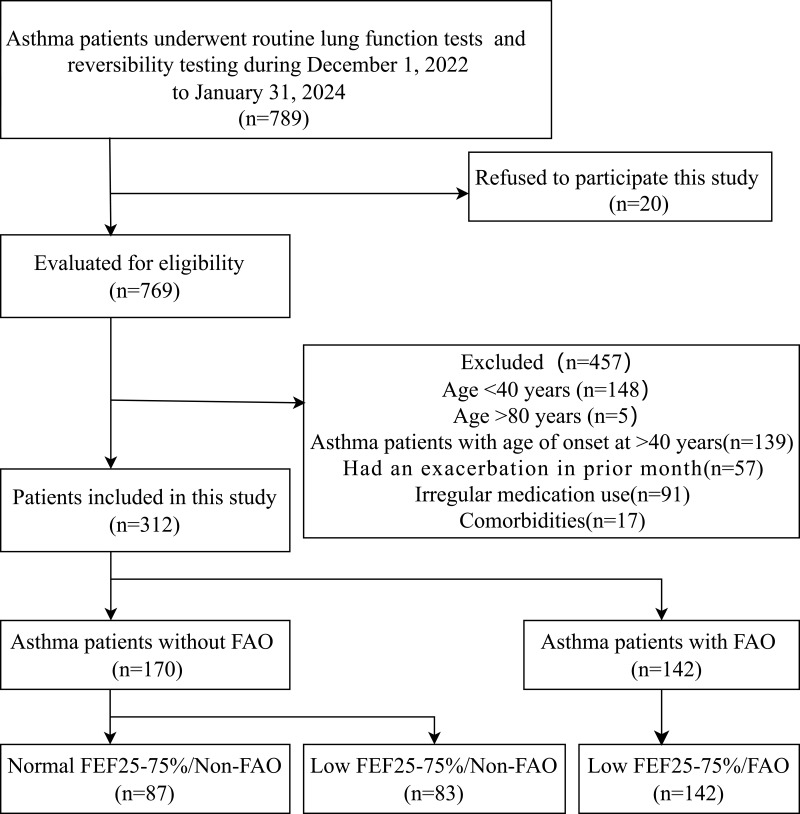
Between December 1, 2022, and January 31, 2024, 789 asthma patients underwent routine pulmonary function testing and reversibility testing at Henan Provincial People’s Hospital, 312 of whom passed the eligibility assessment (see Figure 1, including reasons for exclusion). The mean age was 59.8 years, with a SD of 9.8 years, and 156 participants (50%) were male. Of the male participants, 91 (58.3%) were former or current smokers, whereas only 2 female participants (1.3%) had a history of smoking. Thirty-seven female participants (24%) were exposed to passive smoking, whereas none of the male participants reported such exposure. Of the 312 asthma patients included in the study, 170 (54.5%) were non-FAO patients and 142 (45.5%) were FAO patients. Among the non-FAO patients, 83 (48.8%) had a low FEF25-75%, while all FAO patients had a low FEF25-75%.
Figure 1.
Flow chart of patient selection. FAO, fixed airflow obstruction; FEF25-75%, forced expiratory flow at 25–75%.
Environmental Risk Factors Associated with the Development of Asthmatic FAO
After differential analysis of known and potential determinants of asthmatic FAO development, sex, pack-years, age at asthma onset, duration of asthma, and allergic comorbidities were selected as potential covariates for further analysis (Table 1, P<0.1). We examined the relationship between different exposure levels (from 6 months to 20 years) and the development of asthmatic FAO. Table 2 shows the results of the multivariate logistic regression analysis for the development of FAO after adjusting for covariates. In male patients (n=156), there was a stable linear relationship between pack-years and the risk of developing asthmatic FAO (OR=1.05, 95% CI 1.03–1.07, P<0.001). In female patients (n=154, excluding two smokers), the association between passive smoking and the development of asthmatic FAO was not significant, regardless of how the exposure threshold was adjusted. In the entire study population (n=312), biomass exposure for >20 years was associated with the development of asthmatic FAO (OR=2.65, 95% CI 1.13–6.43, P=0.027). For occupational exposure, even exposure of >10 years was associated with the development of asthmatic FAO (OR=2.01, 95% CI 1.06–3.86, P=0.035).
Table 2.
Multivariate Associations of the Risk of Developing Asthmatic FAO
| Environmental exposure cut-off | < n | ≥ n | OR | 95% CI | P |
| Pack-yearsa,d | |||||
| Continuous | NA | NA | 1.05 | 1.03–1.07 | <0.001 |
| Passive smokingb,d | |||||
| 6 months | 117 | 37 | 1.29 | 0.55–3.00 | 0.558 |
| 10 years | 127 | 27 | 1.52 | 0.59–3.85 | 0.376 |
| 20 years | 139 | 15 | 1.92 | 0.58–6.19 | 0.275 |
| Biomass exposurec,d | |||||
| 6 months | 209 | 103 | 1.70 | 0.96–3.02 | 0.069 |
| 10 years | 256 | 56 | 1.91 | 0.97–3.81 | 0.064 |
| 20 years | 277 | 35 | 2.65 | 1.13–6.43 | 0.027 |
| Occupational exposurec,d | |||||
| 6 months | 233 | 79 | 1.57 | 0.87–2.84 | 0.132 |
| 10 years | 249 | 63 | 2.01 | 1.06–3.86 | 0.035 |
| 20years | 273 | 39 | 2.67 | 1.24–5.91 | 0.013 |
Notes: aOnly in male participants, bOnly in female participants, cAdjusted for sex, pack-years, dAdjusted for age of asthma onset, asthma duration, allergic comorbidities.
Relationships Between FEF25-75% and Other Lung Function Parameters
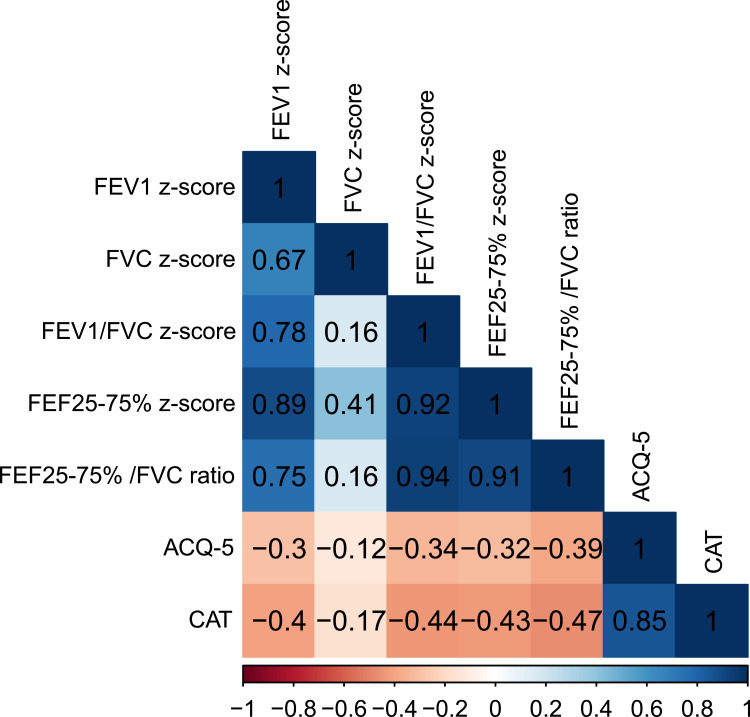
Figure 2 shows the correlation coefficients (r) between different parameters. The correlations between any two variables were all statistically significant (P<0.05). In the entire sample (n=312), the FEF25-75% z-score showed a strong correlation with the FEF25-75%/FVC ratio (r=0.91), indicating that the FEF25-75% z-score was stable and essentially unaffected by the FVC. The FEF25-75% z-score was strongly correlated with both the FEV1 z-score (r=0.89) and the FEV1/FVC z-score (r=0.92) but had a weaker correlation with the FVC z-score (r=0.41), supporting the rationale of classifying asthma patients based on low FEF25-75% and FAO: normal FEF25-75%/FAO−, low FEF25-75%/FAO−, and low FEF25-75%/FAO+, reflecting progressively worsening airflow obstruction severity.
Figure 2.
Correlation matrix diagram of different lung function parameters and questionnaires. FEV1, forced expiratory volume in 1 s; FVC, forced vital capacity; FEF25-75%, forced expiratory flow at 25–75%; ACQ-5, asthma control questionnaire-5; CAT, COPD assessment test.
The Applicability of the CAT to the Assessment of Daily Symptoms in Asthmatics
Figure 2 shows a strong correlation between the CAT and ACQ-5 scores (r=0.85). Table 3 shows that the CAT score had a similar performance to the ACQ-5 score, being highest in the low FEF25-75%/FAO group and lowest in the normal FEF25-75%/NON-FAO asthma group, with significant differences in all multiple comparisons and a trend toward fixed deterioration among the three groups. In addition, there was a stronger association between the CAT score (FEV1 z-score r=0.4, FEV1/FVC z-score r=0.44) and the airflow obstruction parameters than with the ACQ-5 score (FEV1 z-score r=0.3, FEV1/FVC z-score r=0.34) (Figure 2).
Table 3.
Basic Demographic and Disease Characteristics of the Enrolled Participants
| Variable | Total | Normal FEF25-75%/Non-FAO | Low FEF25-75%/Non-FAO | Low FEF25-75%/FAO | P |
|---|---|---|---|---|---|
| n=312 | n=87 | n=83 | n=142 | (P for trend) | |
| Age (years) | 59.8 (9.80) | 60.7 (9.67) | 59.3 (10.3) | 59.6 (9.61) | 0.721 |
| Sex | 0.032 (0.009) | ||||
| Female | 156 (50.0) | 52(59.8) | 44(53.0) | 60(42.3)* | |
| Male | 156 (50.0) | 35 (40.2) | 39 (47.0) | 82 (57.7)* | |
| BMI (kg/m2) | 25.6 [23.0;27.8] | 26.1 [23.3;28.2] | 25.9 [23.5;28.3] | 24.7 [22.9;27.2] | 0.145 |
| Area | 0.100 | ||||
| Urban Area | 162 (51.9) | 47 (54.0) | 50 (60.2) | 65 (45.8) | |
| Rural Area | 150 (48.1) | 40 (46.0) | 33 (39.8) | 77 (54.2) | |
| Age of asthma onset | <0.001 (<0.001) | ||||
| <25 years old | 49 (15.7) | 5 (5.75) | 6 (7.23) | 38 (26.8)*# | |
| ≥25 years old | 263 (84.3) | 82 (94.3) | 77 (92.8) | 104 (73.2)*# | |
| Duration of asthma | |||||
| >25 years | 151 (48.4) | 40 (46.0) | 32 (38.6) | 79 (55.6)# | 0.042 (0.095) |
| >50 years | 19 (6.09) | 2 (2.30) | 3 (3.61) | 14 (9.86) | 0.047 (0.015) |
| Allergic comorbidities | 104 (33.3) | 28 (32.2) | 21 (25.3) | 55 (38.7) | 0.116 |
| CB | 56 (17.9) | 11 (12.6) | 11 (13.3) | 34 (23.9) | 0.046 (0.021) |
| Smoking statusa | 0.084 | ||||
| Never smoker | 65 (41.7) | 20 (57.1) | 19 (48.7) | 26 (31.7) | |
| Current smoker | 42 (26.9) | 6 (17.1) | 8 (20.5) | 28 (34.1) | |
| Ex-smoker | 49 (31.4) | 9 (25.7) | 12 (30.8) | 28 (34.1) | |
| Pack-yearsa | 10.0 [0.00;30.0] | 0.00 [0.00;12.5] | 0.50 [0.00;20.0] | 20.0 [0.00;39.2]*# | 0.001 (0.001) |
| Biomass exposure | |||||
| ≥20 years | 35 (11.2) | 4 (4.60) | 9 (10.8) | 22 (15.5)* | 0.034 (0.011) |
| Occupational exposure | |||||
| ≥10 years | 63 (20.2) | 10 (11.5) | 17 (20.5) | 36 (25.4)* | 0.037 (0.012) |
| ≥20 years | 39 (12.5) | 6 (6.90) | 8 (9.64) | 25 (17.6) | 0.041 (0.013) |
| ACQ-5 | 0.80 [0.40;1.60] | 0.60 [0.40;1.20] | 0.80 [0.60;1.40]* | 1.40 [0.60;2.00]*# | <0.001 (0.001) |
| CAT | 11.5 [7.75;16.0] | 6.00 [5.00;13.0] | 9.00 [8.00;15.5]* | 15.0 [10.0;17.0]*# | <0.001 (0.001) |
| No. of exacerbations in prior y | 0.00 [0.00;1.00] | 0.00 [0.00;1.00] | 0.00 [0.00;1.00] | 1.00 [0.00;1.00]*# | <0.001 (0.001) |
| Had a severe exacerbation in prior y |
55 (17.6) | 9 (10.3) | 12 (14.5) | 34 (23.9)* | 0.025 (0.007) |
Notes: Categorical variables were presented as count (percentage), continuous variables conforming to a normal distribution as mean (SD), and otherwise as median (IQR). aonly in male participants. Depending on the type of variable, the Kruskal‒Wallis test and Fisher’s exact test were used for comparisons among the three groups. If the Kruskal‒Wallis test showed a significant association, the Jonckheere–Terpstra trend test was applied; if Fisher’s exact test showed a significant association, the Mantel–Haenszel trend test was applied. The results of the trend tests were shown in parentheses. *Significantly different from Normal FEF25-75%/ Non-FAO. #Significantly different from Low FEF25-75%/ Non-FAO.
Characteristics of Asthma Patients Classified by Low FEF25-75% and FAO
Regarding medication use, the low FEF25-75%/NON-FAO group had the highest use of ICS (95.2%) and LABA (94%), and the low FEF25-75%/FAO group had the highest use of LAMA (39.4%), whereas there was no difference in LTRA use among the three groups (Table 4). According to the FAO severity classification by the FEV1 z-score, the low FEF25-75%/FAO group was further divided into five groups: mild (23.2%), moderate (13.4%), moderately severe (12.7%), severe (25.4%), and very severe (25.4%) (Table 4).
Table 4.
Lung Function Parameters, Controller Selection, and FAO Severity of Enrolled Participants
| Variable | Variableotal | Normal FEF25-75%/Non-FAO | Low FEF25-75%/ Non-FAO | Low FEF25-75%/ FAO | P |
| n=312 | n=87 | n=83 | n=142 | ||
| Spirometric measures | |||||
| FEV1 z-score | −1.76 [−2.96;–0.58] | −0.03 [−0.64;0.62] | −1.63 [−2.25;–1.10]* | −3.05 [−3.99;–2.08]*# | <0.001 |
| FVC z-score | −0.81 [−1.82;0.13] | −0.02 [−0.83;0.66] | −1.38 [−2.09;–0.58]* | −1.05 [−1.89;–0.15]* | <0.001 |
| FEV1/FVC z-score | −1.50 [−3.46;–0.60] | 0.14 [−0.56;0.62] | −1.17 [−1.45;–0.80]* | −3.57 [−4.20;–2.59]*# | <0.001 |
| FEF25-75% z-score | −1.60 [−2.61;–0.62] | −0.08 [−0.38;0.45] | −1.40 [−1.72;–1.11]* | −2.68 [−3.28;–2.14]*# | <0.001 |
| FEF25-75%/FVC ratio | 41.0 [25.9;59.5] | 73.5 [60.5;90.6] | 46.4 [41.1;54.9]* | 24.8 [17.8;31.7]*# | <0.001 |
| Controller selection | |||||
| ICS | 273 (87.5) | 65 (74.7) | 79 (95.2)* | 129 (90.8)* | <0.001 |
| LABA | 268 (85.9) | 63 (72.4) | 78 (94.0)* | 127 (89.4)* | <0.001 |
| LAMA | 71 (22.8) | 3 (3.45) | 12 (14.5)* | 56 (39.4)*# | <0.001 |
| LTRA | 159 (51.0) | 46 (52.9) | 41 (49.4) | 72 (50.7) | 0.896 |
| FAO severity | |||||
| Mild | 33 (23.2) | ||||
| Moderate | 19 (13.4) | ||||
| Moderately severe | 18 (12.7) | ||||
| Severe | 36 (25.4) | ||||
| Very severe | 36 (25.4) |
Notes: Categorical variables were presented as count (percentage), continuous variables were presented as median (IQR). Severity of FAO were stratified using FEV1 z-score. *Significantly different from Normal FEF25-75%/Non-FAO. #Significantly different from Low FEF25-75%/Non-FAO. See Figure 2 legend for expansion of other abbreviations.
Abbreviations: ICS, inhaled corticosteroids; LABA, long-acting beta-2 agonist; LAMA, long-acting muscarinic antagonist; LTRA, leukotriene receptor antagonist.
Between the normal FEF25-75%/NON-FAO group, the low FEF25-75%/NON-FAO group, and the low FEF25-75%/FAO group, the proportions of males (P=0.032), early-onset asthma (P<0.001), asthma lasting >25 years (P=0.042), asthma lasting >50 years (P=0.047), and CB (P=0.046) differed. As airflow obstruction worsened, there was an increasing trend in the proportions of males (P=0.009 for trend test), early onset of asthma (P<0.001 for trend test), asthma lasting > 50 years (P=0.015 for trend test), and CB (P=0.021 for trend test) (Table 3).
Regarding environmental exposure, the number of pack-years (males, P=0.001) and the proportions of those with biomass exposure for >20 years (P=0.034), occupational exposure for >10 years (P=0.037), and occupational exposure for >20 years (P=0.041) differed among the three groups. With worsening airflow obstruction, the number of pack-years tended to increase (P=0.001 for trend test), and the proportions of biomass exposure for >20 years (P=0.011 for trend test), occupational exposure for >10 years (P=0.012 for trend test), and occupational exposure for >20 years (P=0.013 for trend test) tended to increase (Table 3). Regarding daily symptoms, the ACQ-5 (P<0.001) and CAT scores (P<0.001) differed among the three groups. As airflow obstruction worsened, the patients tended to have higher ACQ-5 (trend test, P=0.001) and CAT scores (trend test, P=0.001) (Table 3). The number of exacerbations in the previous year (P<0.001) and the proportion of severe exacerbations (P=0.025) differed among the three groups. The number of exacerbations in the previous year (P=0.001 for the trend test) and the proportion of severe exacerbations (P=0.007 for the trend test) tended to increase with worsening airflow obstruction (Table 3). Compared with the trend test, the results of the multiple tests were different. The low FEF25-75%/FAO group had significantly greater environmental exposure. Although there was a tendency for the low FEF25-75%/NON-FAO group to have greater environmental exposure than the normal FEF25-75%/NON-FAO group, the difference was not significant. Regarding symptoms, the ACQ-5 and CAT scores showed significant differences among the three groups and a trend toward fixed worsening. Regarding exacerbations, the low FEF25-75%/FAO group had a significantly greater number of exacerbations in the previous year and a greater rate of severe exacerbations, with the number of exacerbations in the previous year being significantly different from both the low FEF25-75%/NON-FAO group and the normal FEF25-75%/NON-FAO group, and the rate of severe exacerbations only being significantly different from the normal FEF25-75%/NON-FAO group. Although the low FEF25-75%/NON-FAO group tended to have more exacerbations than the normal FEF25-75%/NON-FAO group, the difference was not statistically significant.
Discussion
Using data from 312 patients with stable asthma treated with standard controllers, we found that the risk of asthmatic FAO increased with pack-years (in males), biomass exposure years, and occupational exposure years, independent of sex, age of asthma onset, asthma duration, pack-years (for environmental exposures other than smoking), and allergic comorbidities. We found that a 20-year threshold for biomass exposure and a 10-year threshold for occupational exposure were associated with the development of asthmatic FAO. In addition, our finding that asthmatics with FAO had a low FEF25-75% (measured using z-scores), whereas only 48.8% of asthmatics without FAO had a low FEF25-75%, suggests that early small airway impairment represented by low FEF25-75% precedes the development of FAO in asthma. We further showed that low FEF25-75% could be considered an early pathological manifestation before the development of asthmatic FAO. Compared with the normal FEF25-75%/NON-FAO group, the low FEF25-75%/NON-FAO group had poorer lung function, worse daily symptoms, a tendency to have more environmental exposure, and a tendency for more frequent exacerbations, which were further manifested in the asthma group with FAO. These results should be validated through longitudinal studies.
In terms of environmental exposure and exacerbations, there were no significant differences between the low FEF25-75%/NON-FAO group and the normal FEF25-75%/NON-FAO group, which may be due to two reasons: (1) the subgroup of asthmatics with small airway impairment, expressed as low FEF25-75%, reflects the early pathological impairment of FAO that is not significant, and the airway impairments that are reflected do not require significant and prolonged environmental exposure to be mediated; (2) subgrouping by thresholds makes differences between groups less likely to be apparent, and forms of environmental exposure expressed as quantitative data are more likely to show differences, which requires large samples of prospective follow-up data.
Our findings highlight that the development of asthmatic FAO is not only associated with chronic persistent inflammation but also with long-term exposure to adverse environmental factors, such as smoking, biomass smoke, and occupational hazards. GINA1 listed tobacco smoke, toxic chemicals, and occupational exposure as risk factors for asthmatic FAO in 2022; however, further evidence is lacking, and our findings fill this gap. In other words, the development of FAO in asthma may be influenced by multiple mechanisms: part of the disease is mediated by long-term airway inflammation induced by asthma, while the other part is mediated by airway injury caused by prolonged exposure to toxic particles and gases, and there may be a synergistic interaction between these two mechanisms.24–26 Our findings are similar to those of a previous longitudinal population health study conducted in Tasmania, which found that asthma and active smoking were independently associated with the development of FAO in later adulthood and that their interaction (multiplicative effect) led to a more significant post-bronchodilator reduction in FEV1/FVC, especially in atopic individuals.26 In addition, the TESAOD study showed that the likelihood of developing FAO in patients with asthma increased with each 10-pack-year increase in smoking, regardless of whether asthma onset occurred before or after the age of 25 years.27 The association between the development of asthmatic FAO and environmental exposure reported in other studies differs from our findings. A study examining factors associated with FAO in patients with severe asthma found no association between the number of cigarettes smoked and the development of asthmatic FAO; however, the study included only patients with <10 pack-years of smoking and did not consider the effects of other environmental exposures.28 Another study highlighted the effect of occupational exposure on lung function in asthmatics and showed that prolonged occupational exposure could lead to an accelerated decline in FEV1. However, once exposure ceased, the rate of FEV1 decline returned to normal. Despite this finding, with its relatively short follow-up period, the study did not report an association between continuous occupational exposure and asthmatic FAO.29 To date, no studies have confirmed the effect of environmental exposures other than smoking on the development of FAO in asthmatics. In our study, we found an association between the development of asthmatic FAO and biomass as well as occupational exposure, with the risk of developing FAO increasing with the level of occupational exposure.
Our study provides evidence that early small airway damage occurs prior to the development of FAO in asthma and supports the use of FEF25-75% (expressed as a z-score) as a tool to assess the potential risk of FAO development in asthmatics. For ethical reasons, noninvasive techniques are preferred for the indirect assessment of small airway damage.30 Compared to spirometry, impulse oscillometry (IOS) is a noninvasive technique that is more sensitive to small airway function.31 However, to date, no standards and appropriate reference values have been developed for IOS that can be published and adopted worldwide as recommendations, and in addition, IOS is not used as a routine test for asthmatics but only as an alternative when patients are unable to perform spirometry or have contraindications to spirometry.24 The z-score somewhat eliminates the high variability of the FEF25-75% itself, and our study confirms that the FEF25-75% expressed as a z-score correlates well with other measures of lung function and has a high degree of agreement before and after correction for FVC; however, this does not mean that it is the optimal measure of small airway function. No single method is considered the “gold standard” for assessing small airway function, and combined testing may be a more promising solution for assessing small airway function and predicting adverse asthma outcomes.24 When used in combination with FEF25-75%, IOS has been shown to identify more asthmatics with poorer ACQ scores and more frequent exacerbations.32 Quantitative CT measurements can more accurately identify lung parenchymal injury and small airway involvement, which may help scientifically investigate the relative contribution of small airway damage and lung parenchymal injury to asthma FAO.18 The potential role of combined testing in the early identification of FAO in patients with asthma requires further investigation.
In our study, the lung function and clinical characteristics of asthmatics with low FEF25-75% without FAO tended to approach those of asthmatics with FAO, but to a lesser extent. This suggests that compared with FAO, small airway injury represented by low FEF25-75% may represent an early pathological feature of asthmatics with FAO at an earlier stage of the development of asthmatic FAO. Compared to FAO, which is difficult to reverse, small airway damage is more easily intercepted and even reversed and cured,33 which implies the clinical utility of measuring FEF25-75% z-scores in asthmatics.
CAT was previously only used in patients with COPD; however, in recent years, studies have attempted to extend its use to patients with asthma and ACO.22,23 One study attempted to replace disease-specific terms with the generic “pulmonary disease” language and extended the modified questionnaire to asthma and ACO patients to assess the impact of a person’s symptoms on their current level of health, ultimately finding that the modified questionnaire reflected the same health impairments in asthma and ACO patients.23 Another study applied the CAT directly to patients with asthma and ACO and compared it with the ACQ and found that CAT scores correlated well with ACQ scores, especially in the ACO patient group.22 Our study confirmed that CAT scores correlate well with ACQ-5 scores when applied to daily symptom assessment in asthma patients with and without FAO, the group differences in CAT scores were similar to those obseved in ACQ-5 scores in asthma patients categorized by low FEF25-75% and FAO.
Our study has several limitations. First, our participants were recruited from Henan Provincial People’s Hospital, located in northern China, and were all of Han ethnicity, with some from underdeveloped regions. As this was a single-center study with a relatively small sample size, the generalizability of the results to the entire asthma population is somewhat limited. Second, the self-reported environmental exposure was unreliable. Environmental exposure self-reported by participants may be biased owing to patients’ recall bias, subjectivity, and comprehension. Several approaches were used to minimize these deficiencies, including designing the questions to be more accessible, increasing privacy during the interview, allowing patients to report more freely about what actually happened, and providing broader thresholds to reduce the pressure on patients to make choices. Ideally, we should directly select asthmatic children for follow-up to obtain reliable exposure history data, which requires further large-scale long-term follow-up. Third, to exclude as many patients as possible with pure COPD and those in whom FAO preceded the onset of asthma, the following efforts were made: (1) We required patients to have an age of asthma onset <40 years. A systematic review and modeling study showed that the prevalence of COPD (defined as FEV1/FVC < LLN) in people aged <40 years was <4.9% and limiting the age of onset to 40 years could largely minimize the erroneous inclusion of patients with COPD.34 However, this may have biased our reported number of exacerbations in the previous year and the rate of severe exacerbations in the overall asthma population, as asthmatics with disease onset after 40 years of age have been shown to have more frequent exacerbations.35 (2) We required that patients had a previous diagnosis of asthma confirmed by a positive lung function provocation test or a positive bronchodilator reversibility test and that patients had symptoms that varied over time and intensity in the early stages of the disease. (3) We excluded patients treated with bronchodilators alone. Nevertheless, these efforts cannot compensate for the shortcomings of retrospective studies. Long-term lung function follow-up of patients with pure asthma is the best approach for exploring the etiology and risk factors for the development of asthmatic FAO. Fourth, owing to the retrospective nature of the study, we did not consider the interference of asthma severity with the type of asthmatic inflammation in the results. In a previous study, asthma severity was confirmed to be independent of airway remodeling (pathological changes in asthmatic FAO).36 Current evidence regarding the relationship between airway eosinophil or neutrophil inflammation levels and FAO in asthmatics is conflicting.2 Fifth, we did not account for lung function injuries caused by air pollution, because air quality varies over time and across different geographic regions. In addition, it was difficult to ascertain the subjects’ outdoor activities over the past decades and their complex geographic mobility patterns. Nevertheless, it has been shown that asthma patients exposed to high levels of air pollution are more likely to progress to FAO.37 Furthermore, we did not consider the potential interactions between different environmental exposures other than smoking, which may have influenced our results.
Conclusion
In conclusion, management and treatment targeting environmental risk factors (smoking and biomass and occupational exposures) may help delay the development of FAO in asthmatics. FEF25-75% measured by z-score reliably identifies the subgroup of asthmatics with abnormal small airway function who are at a higher risk of FAO and should therefore be followed more closely. The small airway may be the earliest site of FAO in asthma, and the timely reversal of small airway damage or prevention of further small airway damage may be future research directions.
Funding Statement
The study was funded by the Health Commission of Henan Province (No. SBGJ202302003), but the authors designed the study, recruited the study participants to collect the data, analyzed and interpreted the data and wrote the manuscript.
Abbreviations
FAO, Fixed airflow obstruction; FEF25-75%, Forced expiratory flow at 25-75%; FEV1, Forced expiratory volume in 1 s; FVC, Forced vital capacity; COPD, Chronic obstructive pulmonary disease; CB, Chronic bronchitis; ACQ-5, Asthma Control Questionnaire-5; CAT, COPD Assessment Test; BD, Bronchodilation; OR, Odds ratio; SD, Standard deviation; IQR, Interquartile range; r, correlation coefficients; ICS, Inhaled corticosteroids; LABA, Long-acting beta-2 agonist; LAMA, Long-acting muscarinic antagonist; LTRA, Leukotriene receptor antagonist.
Data Sharing Statement
The datasets utilized and/or examined in the present study can be obtained from the corresponding author upon a reasonable request.
Ethics Approval and Informed Consent
The study was approved by the Medical Ethics Committee of Henan Provincial People’s Hospital, and every patient provided written informed consent.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Global Initiative for Asthma. Global strategy for asthma management and prevention, 2022. Available from: www.ginasthma.org. Accessed October 4, 2024.
- 2.Rutting S, Tonga KO, King GG. Toward explaining fixed airflow obstruction in asthma. J Allergy Clin Immunol. 2022;149(3):890–892. doi: 10.1016/j.jaci.2021.12.784 [DOI] [PubMed] [Google Scholar]
- 3.Lin H, Li H. How does cigarette smoking affect airway remodeling in asthmatics? Tob Induc Dis. 2023;21:13. doi: 10.18332/tid/156047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.de la Hoz RE, Shapiro M, Nolan A, et al. Association of world trade center (WTC) occupational exposure intensity with chronic obstructive pulmonary disease (COPD) and asthma COPD overlap (ACO). Lung. 2023;201(4):325–334. doi: 10.1007/s00408-023-00636-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pellegrino R, Viegi G, Brusasco V, et al. Interpretative strategies for lung function tests. Europ Resp J. 2005;26(5):948–968. doi: 10.1183/09031936.05.00035205 [DOI] [PubMed] [Google Scholar]
- 6.Global strategy for prevention, diagnosis and management of COPD: 2023 report. 2022. Available from: https://goldcopd.org/2023-gold-report-2/. Accessed October 4, 2024.
- 7.Quanjer PH, Pretto JJ, Brazzale DJ, Boros PW. Grading the severity of airways obstruction: new wine in new bottles. Europ Resp J. 2014;43(2):505–512. doi: 10.1183/09031936.00086313 [DOI] [PubMed] [Google Scholar]
- 8.Quanjer PH, Stanojevic S, Cole TJ, et al. Multi-ethnic reference values for spirometry for the 3-95-yr age range: the global lung function 2012 equations. Europ Resp J. 2012;40(6):1324–1343. doi: 10.1183/09031936.00080312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fransen LFH, Leonard MO. Small airway susceptibility to chemical and particle injury. Respiration. 2022;101(3):321–333. doi: 10.1159/000519344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Alobaidi NY, Almeshari M, Stockley J, Stockley RA, Sapey E. Small airway function measured using forced expiratory flow between 25% and 75% of vital capacity and its relationship to airflow limitation in symptomatic ever-smokers: a cross-sectional study. BMJ Open Respir Res. 2022;9(1):e001385. doi: 10.1136/bmjresp-2022-001385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kwon DS, Choi YJ, Kim TH, et al. FEF(25-75%) values in patients with normal lung function can predict the development of chronic obstructive pulmonary disease. Int J Chronic Obstr. 2020;15:2913–2921. doi: 10.2147/copd.S261732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xue Y, Bao W, Zhou Y, et al. Small-airway dysfunction is involved in the pathogenesis of asthma: evidence from two mouse models. J Asthma Allergy. 2021;14:883–896. doi: 10.2147/jaa.S312361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Konstantinos Katsoulis K, Kostikas K, Kontakiotis T. Techniques for assessing small airways function: possible applications in asthma and COPD. Respir Med. 2016;119:e2–e9. doi: 10.1016/j.rmed.2013.05.003 [DOI] [PubMed] [Google Scholar]
- 14.Hansen JE, Sun XG, Wasserman K. Discriminating measures and normal values for expiratory obstruction. Chest. 2006;129(2):369–377. doi: 10.1378/chest.129.2.369 [DOI] [PubMed] [Google Scholar]
- 15.Alsayed AR, Abu-Samak MS, Alkhatib M. Asthma-COPD overlap in clinical practice (ACO_CP 2023): toward precision medicine. J Personalized Med. 2023;13(4):677. doi: 10.3390/jpm13040677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mekov E, Nuñez A, Sin DD, et al. Update on asthma-COPD overlap (ACO): a narrative review. Int J Chronic Obstr. 2021;16:1783–1799. doi: 10.2147/copd.S312560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dharmage SC, Perret JL, Burgess JA, et al. Current asthma contributes as much as smoking to chronic bronchitis in middle age: a prospective population-based study. Int J Chronic Obstr. 2016;11:1911–1920. doi: 10.2147/copd.S103908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shimizu K, Tanabe N, Oguma A, et al. Parenchymal destruction in asthma: fixed airflow obstruction and lung function trajectory. J Allergy Clin Immunol. 2022;149(3):934–942.e8. doi: 10.1016/j.jaci.2021.07.042 [DOI] [PubMed] [Google Scholar]
- 19.McGeachie MJ, Yates KP, Zhou X, et al. Patterns of growth and decline in lung function in persistent childhood asthma. New Engl J Med. 2016;374(19):1842–1852. doi: 10.1056/NEJMoa1513737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lira G, da Silva GAP, Bezerra PGM, Sarinho ESC. Avoidance of inhaled pollutants and irritants in asthma from a salutogenic perspective. J Asthma Allergy. 2024;17:237–250. doi: 10.2147/jaa.S445864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Juniper EF, Svensson K, Mörk AC, Ståhl E. Measurement properties and interpretation of three shortened versions of the asthma control questionnaire. Respir Med. 2005;99(5):553–558. doi: 10.1016/j.rmed.2004.10.008 [DOI] [PubMed] [Google Scholar]
- 22.Kurashima K, Takaku Y, Ohta C, Takayanagi N, Yanagisawa T, Sugita Y. COPD assessment test and severity of airflow limitation in patients with asthma, COPD, and asthma-COPD overlap syndrome. Int J Chronic Obstr. 2016;11:479–487. doi: 10.2147/copd.S97343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tomaszewski EL, Atkinson MJ, Janson C, et al. Chronic Airways Assessment Test: psychometric properties in patients with asthma and/or COPD. Respir Res. 2023;24(1):106. doi: 10.1186/s12931-023-02394-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Banno A, Reddy AT, Lakshmi SP, Reddy RC. Bidirectional interaction of airway epithelial remodeling and inflammation in asthma. Clin Sci. 2020;134(9):1063–1079. doi: 10.1042/cs20191309 [DOI] [PubMed] [Google Scholar]
- 25.Kayalar Ö, Rajabi H, Konyalilar N, et al. Impact of particulate air pollution on airway injury and epithelial plasticity; underlying mechanisms. Front Immunol. 2024;15:1324552. doi: 10.3389/fimmu.2024.1324552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Perret JL, Dharmage SC, Matheson MC, et al. The interplay between the effects of lifetime asthma, smoking, and atopy on fixed airflow obstruction in middle age. Am J Respir Crit Care Med. 2013;187(1):42–48. doi: 10.1164/rccm.201205-0788OC [DOI] [PubMed] [Google Scholar]
- 27.Guerra S, Sherrill DL, Kurzius-Spencer M, et al. The course of persistent airflow limitation in subjects with and without asthma. Respir Med. 2008;102(10):1473–1482. doi: 10.1016/j.rmed.2008.04.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.ten Brinke A, Zwinderman AH, Sterk PJ, Rabe KF, Bel EH. Factors associated with persistent airflow limitation in severe asthma. Am J Respir Crit Care Med. 2001;164(5):744–748. doi: 10.1164/ajrccm.164.5.2011026 [DOI] [PubMed] [Google Scholar]
- 29.Anees W, Moore VC, Burge PS. FEV1 decline in occupational asthma. Thorax. 2006;61(9):751–755. doi: 10.1136/thx.2005.054080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Contoli M, Santus P, Papi A. Small airway disease in asthma: pathophysiological and diagnostic considerations. Curr Opin Pulm Med. 2015;21(1):68–73. doi: 10.1097/mcp.0000000000000122 [DOI] [PubMed] [Google Scholar]
- 31.Bednarek M, Grabicki M, Piorunek T, Batura-Gabryel H. Current place of impulse oscillometry in the assessment of pulmonary diseases. Respir Med. 2020;170:105952. doi: 10.1016/j.rmed.2020.105952 [DOI] [PubMed] [Google Scholar]
- 32.Chan R, Lipworth B. Interactions between spirometry and oscillometry in patients with moderate to severe asthma. Europ Resp J. 2022;60(4):2200543. doi: 10.1183/13993003.00543-2022 [DOI] [PubMed] [Google Scholar]
- 33.Cosio M, Ghezzo H, Hogg JC, et al. The relations between structural changes in small airways and pulmonary-function tests. New Engl J Med. 1978;298(23):1277–1281. doi: 10.1056/nejm197806082982303 [DOI] [PubMed] [Google Scholar]
- 34.Adeloye D, Song P, Zhu Y, Campbell H, Sheikh A, Rudan I. Global, regional, and national prevalence of, and risk factors for, chronic obstructive pulmonary disease (COPD) in 2019: a systematic review and modelling analysis. Lancet Respir Med. 2022;10(5):447–458. doi: 10.1016/s2213-2600(21)00511-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Baan EJ, de Roos EW, Engelkes M, et al. Characterization of asthma by age of onset: a multi-database cohort study. J Allergy Clini Immunol Pract. 2022;10(7):1825–1834.e8. doi: 10.1016/j.jaip.2022.03.019 [DOI] [PubMed] [Google Scholar]
- 36.Broekema M, Timens W, Vonk JM, et al. Persisting remodeling and less airway wall eosinophil activation in complete remission of asthma. Am J Respir Crit Care Med. 2011;183(3):310–316. doi: 10.1164/rccm.201003-0494OC [DOI] [PubMed] [Google Scholar]
- 37.To T, Zhu J, Larsen K, et al. Progression from asthma to chronic obstructive pulmonary disease. is air pollution a risk factor? Am J Respir Crit Care Med. 2016;194(4):429–438. doi: 10.1164/rccm.201510-1932OC [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets utilized and/or examined in the present study can be obtained from the corresponding author upon a reasonable request.