Abstract
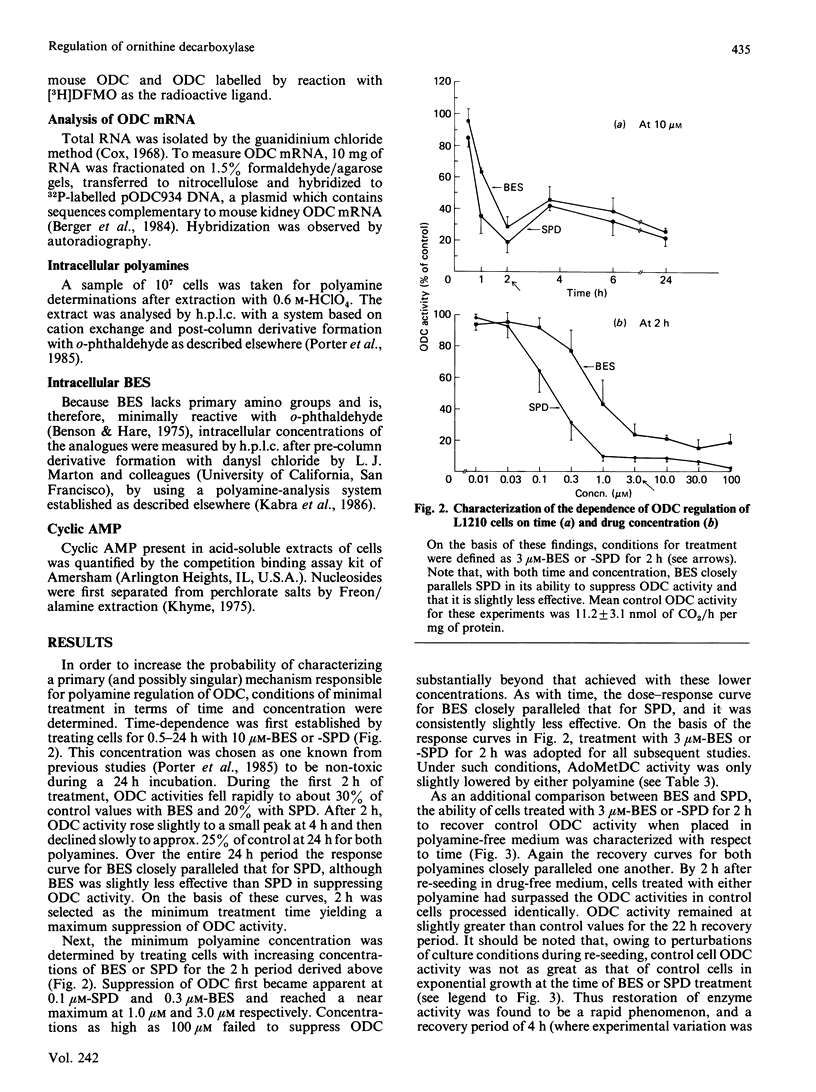
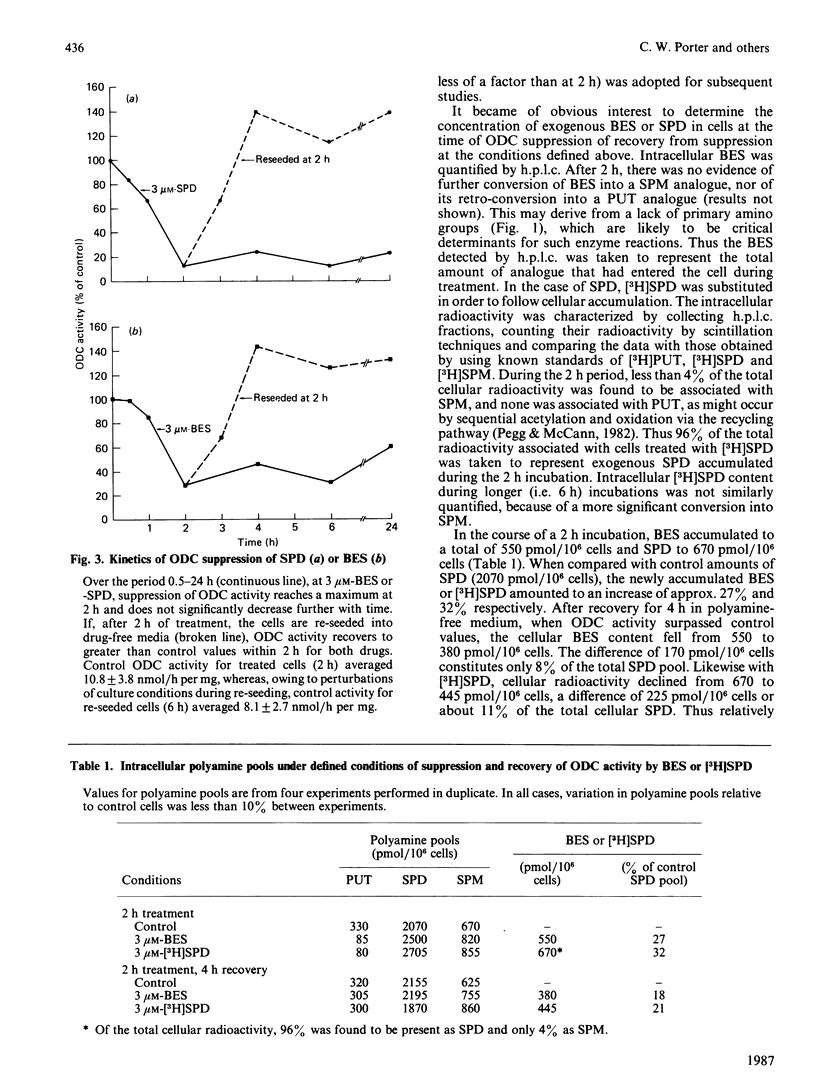
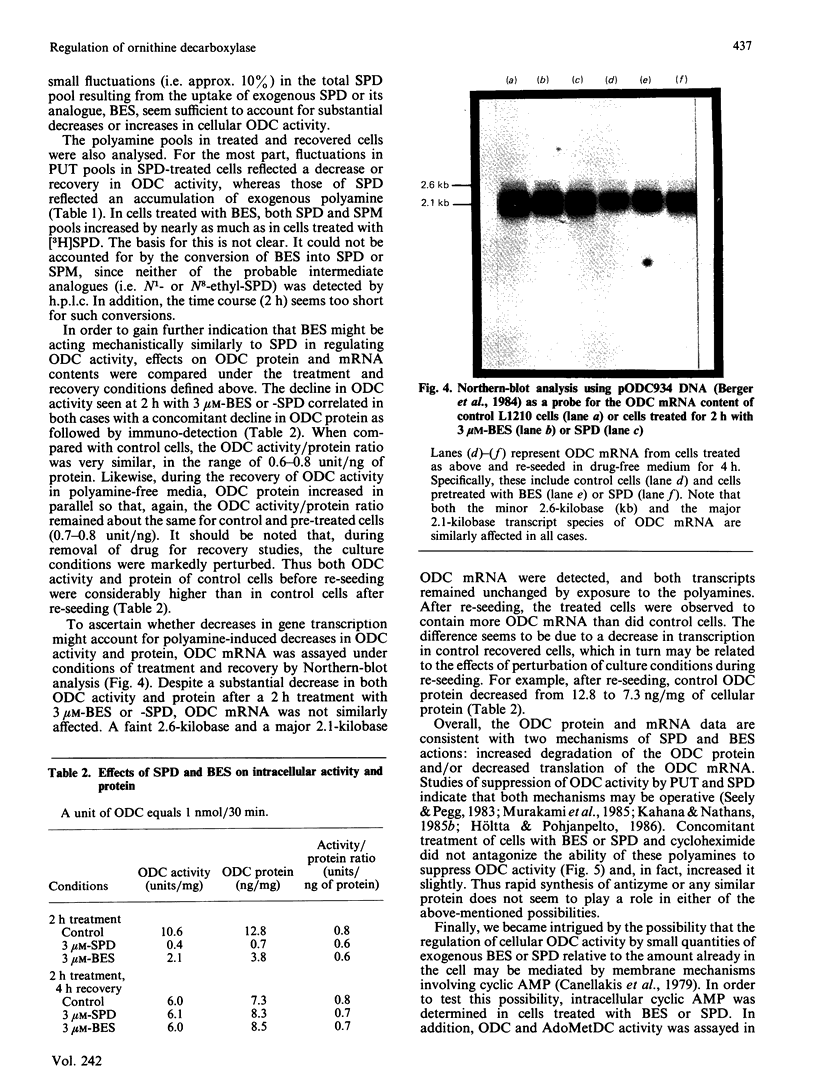
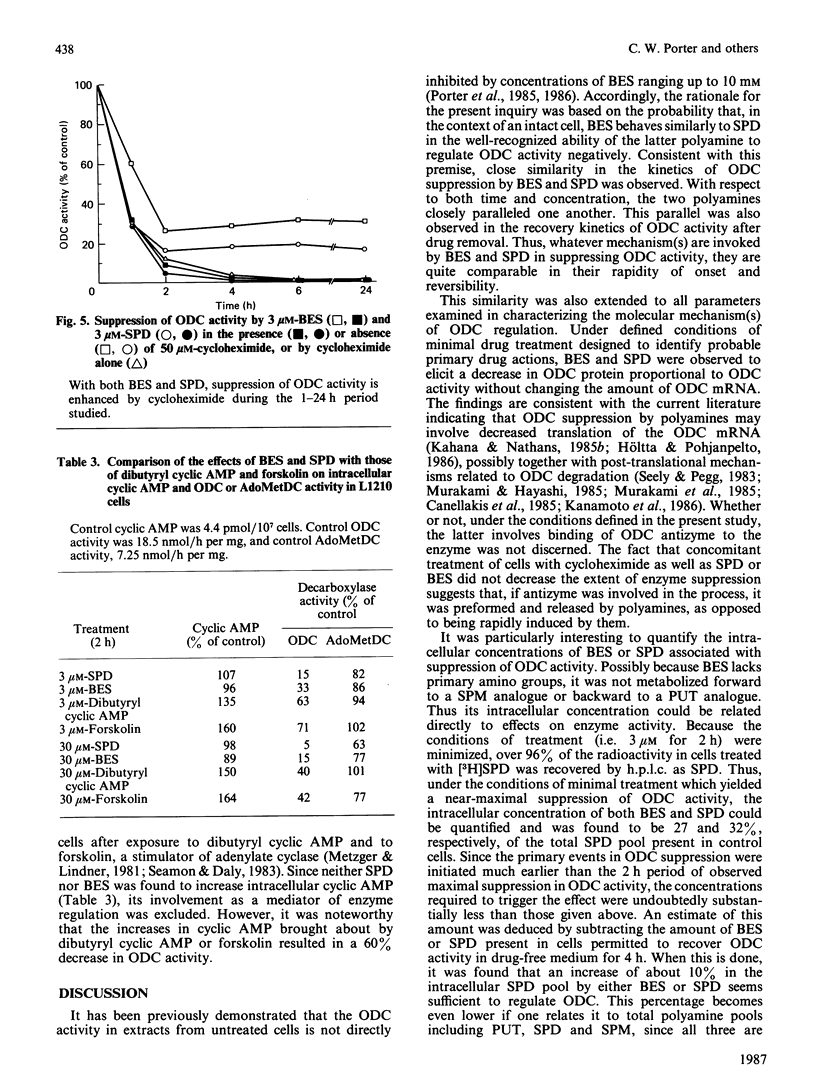
Polyamine biosynthesis in intact cells can be exquisitely controlled with exogenous polyamines through the regulation of rate-limiting biosynthetic enzymes, particularly ornithine decarboxylase (ODC). In an attempt to exploit this phenomenon as an antiproliferative strategy, certain polyamine analogues have been identified [Porter, Cavanaugh, Stolowich, Ganis, Kelly & Bergeron (1985) Cancer Res. 45, 2050-2057] which lower ODC activity in intact cells, have no direct inhibitory effects on ODC, are incapable of substituting for spermidine (SPD) in supporting cell growth, and are growth-inhibitory at micromolar concentrations. In the present study, the most effective of these analogues, N1N8-bis(ethyl)SPD (BES), is compared with SPD in its ability to regulate ODC activity in intact L1210 cells and in the mechanism(s) by which this is accomplished. With respect to time and dose-dependence of ODC suppression, both polyamines closely paralleled one another in their response curves, although BES was slightly less effective than SPD. Conditions of minimal treatment leading to near-maximal ODC suppression (70-80%) were determined and found to be 3 microM for 2 h with either SPD or BES. After such treatment, ODC activity was fully recovered within 2-4 h when cells were re-seeded in drug-free media. By assessing BES or [3H]SPD concentrations in treated and recovered cells, it was possible to deduce that an intracellular accumulation of BES or SPD equivalent to less than 6.5% of the combined cellular polyamine pool was sufficient to invoke ODC regulatory mechanisms. Decreases in ODC activity after BES or SPD treatment were closely paralleled by concomitant decreases in ODC protein. Since cellular ODC mRNA was not similarly decreased by either BES or SPD, it was concluded that translational and/or post-translational mechanisms, such as increased degradation of ODC protein or decreased translation of ODC mRNA, were probably responsible for regulation of enzyme activity. Experimental evidence indicated that neither of these mechanisms seemed to be mediated by cyclic AMP or ODC-antizyme induction. On the basis of the consistent similarities between BES and SPD in all parameters studied, it is concluded that the analogue most probably acts by the same mechanisms as SPD in regulating polyamine biosynthesis.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alhonen-Hongisto L., Seppänen P., Jänne J. Methylglyoxal bis(guanylhydrazone) stimulates the cellular transport system of the polyamines. FEBS Lett. 1982 Aug 23;145(2):182–186. doi: 10.1016/0014-5793(82)80163-4. [DOI] [PubMed] [Google Scholar]
- Atmar V. J., Kuehn G. D. Phosphorylation of ornithine decarboxylase by a polyamine-dependent protein kinase. Proc Natl Acad Sci U S A. 1981 Sep;78(9):5518–5522. doi: 10.1073/pnas.78.9.5518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benson J. R., Hare P. E. O-phthalaldehyde: fluorogenic detection of primary amines in the picomole range. Comparison with fluorescamine and ninhydrin. Proc Natl Acad Sci U S A. 1975 Feb;72(2):619–622. doi: 10.1073/pnas.72.2.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger F. G., Szymanski P., Read E., Watson G. Androgen-regulated ornithine decarboxylase mRNAs of mouse kidney. J Biol Chem. 1984 Jun 25;259(12):7941–7946. [PubMed] [Google Scholar]
- Canellakis E. S., Kyriakidis D. A., Rinehart C. A., Jr, Huang S. C., Panagiotidis C., Fong W. F. Regulation of polyamine biosynthesis by antizyme and some recent developments relating the induction of polyamine biosynthesis to cell growth. Review. Biosci Rep. 1985 Mar;5(3):189–204. doi: 10.1007/BF01119588. [DOI] [PubMed] [Google Scholar]
- Canellakis E. S., Viceps-Madore D., Kyriakidis D. A., Heller J. S. The regulation and function of ornithine decarboxylase and of the polyamines. Curr Top Cell Regul. 1979;15:155–202. [PubMed] [Google Scholar]
- Canellakis Z. N., Theoharides T. C. Stimulation of ornithine decarboxylase synthesis and its control by polyamines in regenerating rat liver and cultured rat hepatoma cells. J Biol Chem. 1976 Jul 25;251(14):4436–4441. [PubMed] [Google Scholar]
- Clark J. L., Fuller J. L. Regulation of ornithine decarboxylase in 3T3 cells by putrescine and spermidine: indirect evidence for translational control. Biochemistry. 1975 Oct 7;14(20):4403–4409. doi: 10.1021/bi00691a010. [DOI] [PubMed] [Google Scholar]
- Fong W. F., Heller J. S., Canellakis E. S. The appearance of an ornithine decarboxylase inhibitory protein upon the addition of putrescine to cell cultures. Biochim Biophys Acta. 1976 Apr 23;428(2):456–465. doi: 10.1016/0304-4165(76)90054-4. [DOI] [PubMed] [Google Scholar]
- Gupta M., Coffino P. Mouse ornithine decarboxylase. Complete amino acid sequence deduced from cDNA. J Biol Chem. 1985 Mar 10;260(5):2941–2944. [PubMed] [Google Scholar]
- Heller J. S., Canellakis E. S. Cellular control of ornithine decarboxylase activity by its antizyme. J Cell Physiol. 1981 May;107(2):209–217. doi: 10.1002/jcp.1041070206. [DOI] [PubMed] [Google Scholar]
- Heller J. S., Fong W. F., Canellakis E. S. Induction of a protein inhibitor to ornithine decarboxylase by the end products of its reaction. Proc Natl Acad Sci U S A. 1976 Jun;73(6):1858–1862. doi: 10.1073/pnas.73.6.1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hölttä E., Pohjanpelto P. Control of ornithine decarboxylase in Chinese hamster ovary cells by polyamines. Translational inhibition of synthesis and acceleration of degradation of the enzyme by putrescine, spermidine, and spermine. J Biol Chem. 1986 Jul 15;261(20):9502–9508. [PubMed] [Google Scholar]
- Kahana C., Nathans D. Nucleotide sequence of murine ornithine decarboxylase mRNA. Proc Natl Acad Sci U S A. 1985 Mar;82(6):1673–1677. doi: 10.1073/pnas.82.6.1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahana C., Nathans D. Translational regulation of mammalian ornithine decarboxylase by polyamines. J Biol Chem. 1985 Dec 15;260(29):15390–15393. [PubMed] [Google Scholar]
- Kallio A., Pösö H., Scalabrino G., Jänne J. Regulation of ornithine decarboxylase by diamines in regenerating rat liver. FEBS Lett. 1977 Feb 1;73(2):229–234. doi: 10.1016/0014-5793(77)80987-3. [DOI] [PubMed] [Google Scholar]
- Kanamoto R., Utsunomiya K., Kameji T., Hayashi S. Effects of putrescine on synthesis and degradation of ornithine decarboxylase in primary cultured hepatocytes. Eur J Biochem. 1986 Feb 3;154(3):539–544. doi: 10.1111/j.1432-1033.1986.tb09432.x. [DOI] [PubMed] [Google Scholar]
- Kay J. E., Lindsay V. J. Control of ornithine decarboxylase activity in stimulated human lymphocytes by putrescine and spermidine. Biochem J. 1973 Apr;132(4):791–796. doi: 10.1042/bj1320791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khym J. X. An analytical system for rapid separation of tissue nucleotides at low pressures on conventional anion exchangers. Clin Chem. 1975 Aug;21(9):1245–1252. [PubMed] [Google Scholar]
- Mamont P. S., Danzin C., Wagner J., Siat M., Joder-Ohlenbusch A. M., Claverie N. Accumulation of decarboxylated S-adenosyl-L-methionine in mammalian cells as a consequence of the inhibition of putrescine biosynthesis. Eur J Biochem. 1982 Apr;123(3):499–504. doi: 10.1111/j.1432-1033.1982.tb06559.x. [DOI] [PubMed] [Google Scholar]
- Mamont P. S., Duchesne M. C., Grove J., Bey P. Anti-proliferative properties of DL-alpha-difluoromethyl ornithine in cultured cells. A consequence of the irreversible inhibition of ornithine decarboxylase. Biochem Biophys Res Commun. 1978 Mar 15;81(1):58–66. doi: 10.1016/0006-291x(78)91630-3. [DOI] [PubMed] [Google Scholar]
- McCann P. P., Tardif C., Hornsperger J. M., Böhlen P. Two distinct mechanisms for ornithine decarboxylase regulation by polyamines in rat hepatoma cells. J Cell Physiol. 1979 May;99(2):183–190. doi: 10.1002/jcp.1040990204. [DOI] [PubMed] [Google Scholar]
- McCann P. P., Tardif C., Mamont P. S. Regulation of ornithine decarboxylase by ODC-antizyme in HTC cells. Biochem Biophys Res Commun. 1977 Apr 25;75(4):948–954. doi: 10.1016/0006-291x(77)91474-7. [DOI] [PubMed] [Google Scholar]
- Metzger H., Lindner E. The positive inotropic-acting forskolin, a potent adenylate cyclase activator. Arzneimittelforschung. 1981;31(8):1248–1250. [PubMed] [Google Scholar]
- Mitchell J. L., Qasba P., Stofko R. E., Franzen M. A. Ornithine decarboxylase modification and polyamine-stimulated enzyme inactivation in HTC cells. Biochem J. 1985 Jun 1;228(2):297–308. doi: 10.1042/bj2280297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell J. L., Sedory M. J. Cycloheximide induced in vivo modification of ornithine decarboxylase in Physarum polycephalum. FEBS Lett. 1974 Dec 1;49(1):120–124. doi: 10.1016/0014-5793(74)80646-0. [DOI] [PubMed] [Google Scholar]
- Morris D. R., Fillingame R. H. Regulation of amino acid decarboxylation. Annu Rev Biochem. 1974;43(0):303–325. doi: 10.1146/annurev.bi.43.070174.001511. [DOI] [PubMed] [Google Scholar]
- Murakami Y., Fujita K., Kameji T., Hayashi S. Accumulation of ornithine decarboxylase-antizyme complex in HMOA cells. Biochem J. 1985 Feb 1;225(3):689–697. doi: 10.1042/bj2250689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murakami Y., Hayashi S. Role of antizyme in degradation of ornithine decarboxylase in HTC cells. Biochem J. 1985 Mar 15;226(3):893–896. doi: 10.1042/bj2260893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pegg A. E., McCann P. P. Polyamine metabolism and function. Am J Physiol. 1982 Nov;243(5):C212–C221. doi: 10.1152/ajpcell.1982.243.5.C212. [DOI] [PubMed] [Google Scholar]
- Pegg A. E., Pösö H., Shuttleworth K., Bennett R. A. Effect of inhibition of polyamine synthesis on the content of decarboxylated S-adenosylmethionine. Biochem J. 1982 Feb 15;202(2):519–526. doi: 10.1042/bj2020519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pera P. J., Kramer D. L., Sufrin J. R., Porter C. W. Comparison of the biological effects of four irreversible inhibitors of ornithine decarboxylase in two murine lymphocytic leukemia cell lines. Cancer Res. 1986 Mar;46(3):1148–1154. [PubMed] [Google Scholar]
- Porter C. W., Bergeron R. J., Stolowich N. J. Biological properties of N4-spermidine derivatives and their potential in anticancer chemotherapy. Cancer Res. 1982 Oct;42(10):4072–4078. [PubMed] [Google Scholar]
- Porter C. W., Cavanaugh P. F., Jr, Stolowich N., Ganis B., Kelly E., Bergeron R. J. Biological properties of N4- and N1,N8-spermidine derivatives in cultured L1210 leukemia cells. Cancer Res. 1985 May;45(5):2050–2057. [PubMed] [Google Scholar]
- Porter C. W., Ganis B., Vinson T., Marton L. J., Kramer D. L., Bergeron R. J. Comparison and characterization of growth inhibition in L1210 cells by alpha-difluoromethylornithine, an inhibitor of ornithine decarboxylase, and N1,N8-bis(ethyl)spermidine, an apparent regulator of the enzyme. Cancer Res. 1986 Dec;46(12 Pt 1):6279–6285. [PubMed] [Google Scholar]
- Porter C. W., Sufrin J. R. Interference with polyamine biosynthesis and/or function by analogs of polyamines or methionine as a potential anticancer chemotherapeutic strategy. Anticancer Res. 1986 Jul-Aug;6(4):525–542. [PubMed] [Google Scholar]
- Russell D. H. Posttranslational modification of ornithine decarboxylase by its product putrescine. Biochem Biophys Res Commun. 1981 Apr 30;99(4):1167–1172. doi: 10.1016/0006-291x(81)90741-5. [DOI] [PubMed] [Google Scholar]
- Seely J. E., Pegg A. E. Effect of 1,3-diaminopropane on ornithine decarboxylase enzyme protein in thioacetamide-treated rat liver. Biochem J. 1983 Dec 15;216(3):701–707. doi: 10.1042/bj2160701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seely J. E., Pegg A. E. Ornithine decarboxylase (mouse kidney). Methods Enzymol. 1983;94:158–161. doi: 10.1016/s0076-6879(83)94025-9. [DOI] [PubMed] [Google Scholar]



