Abstract
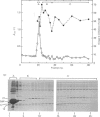
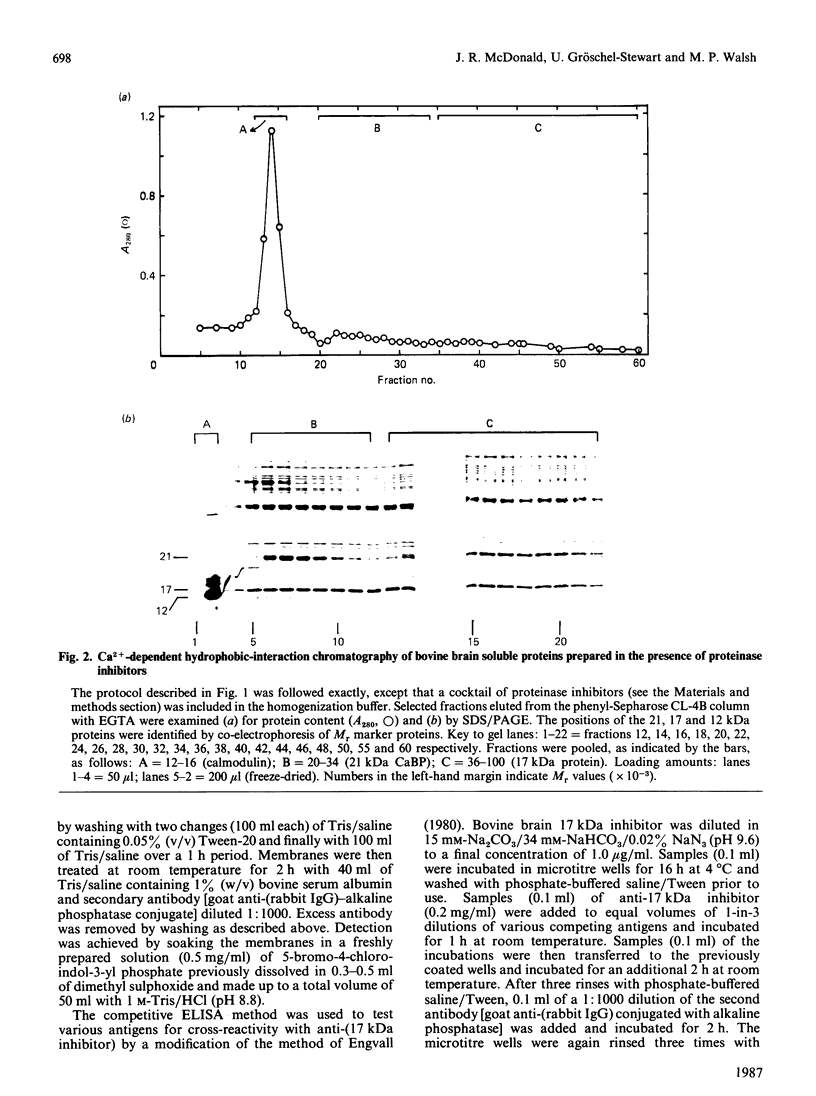
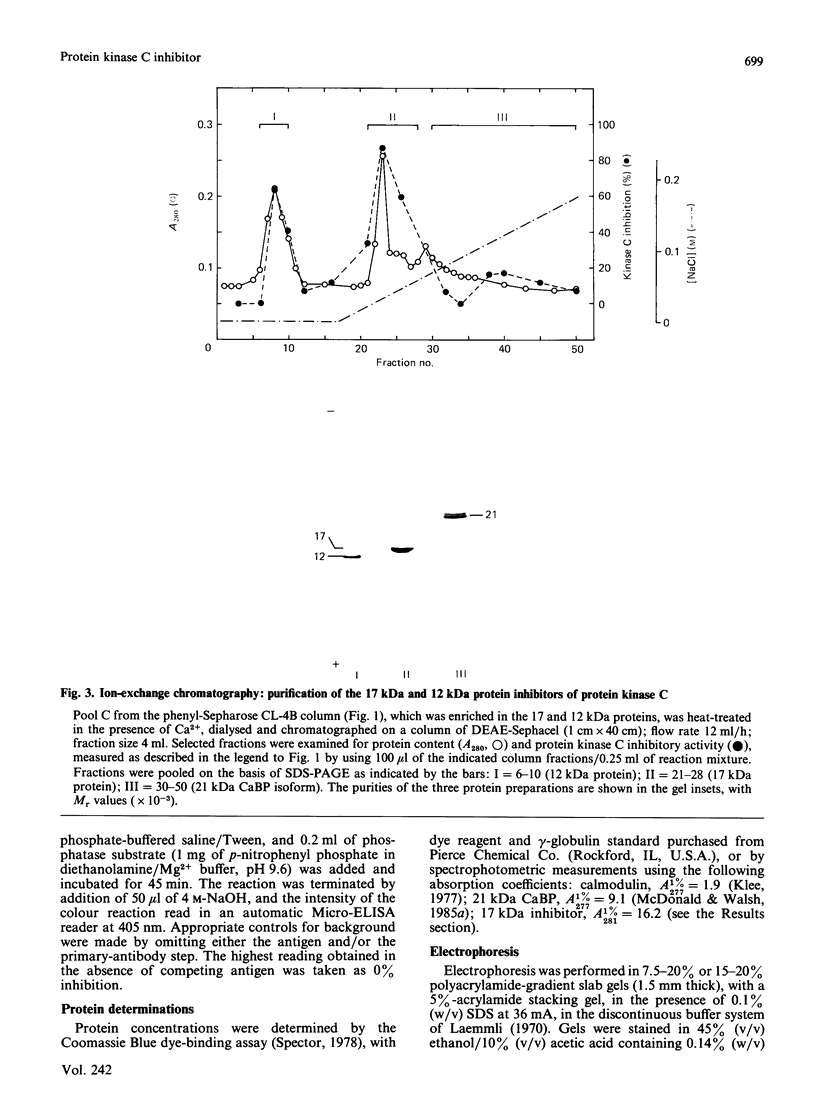
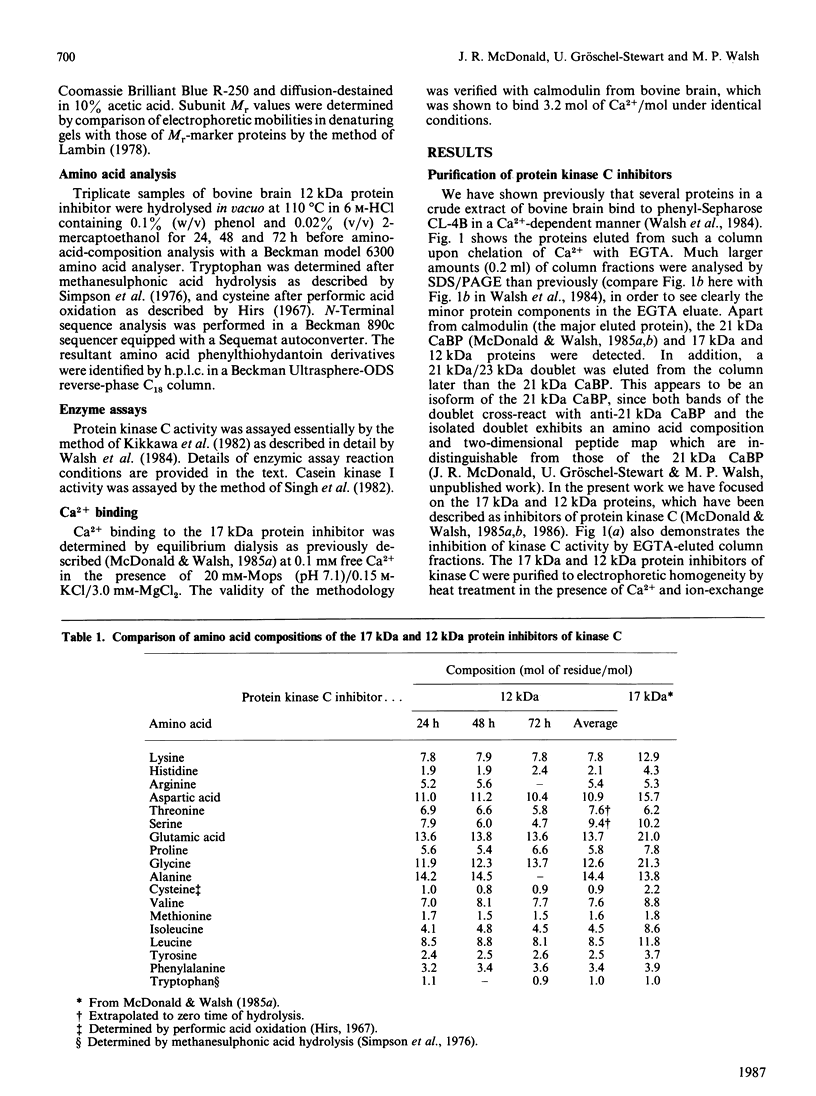
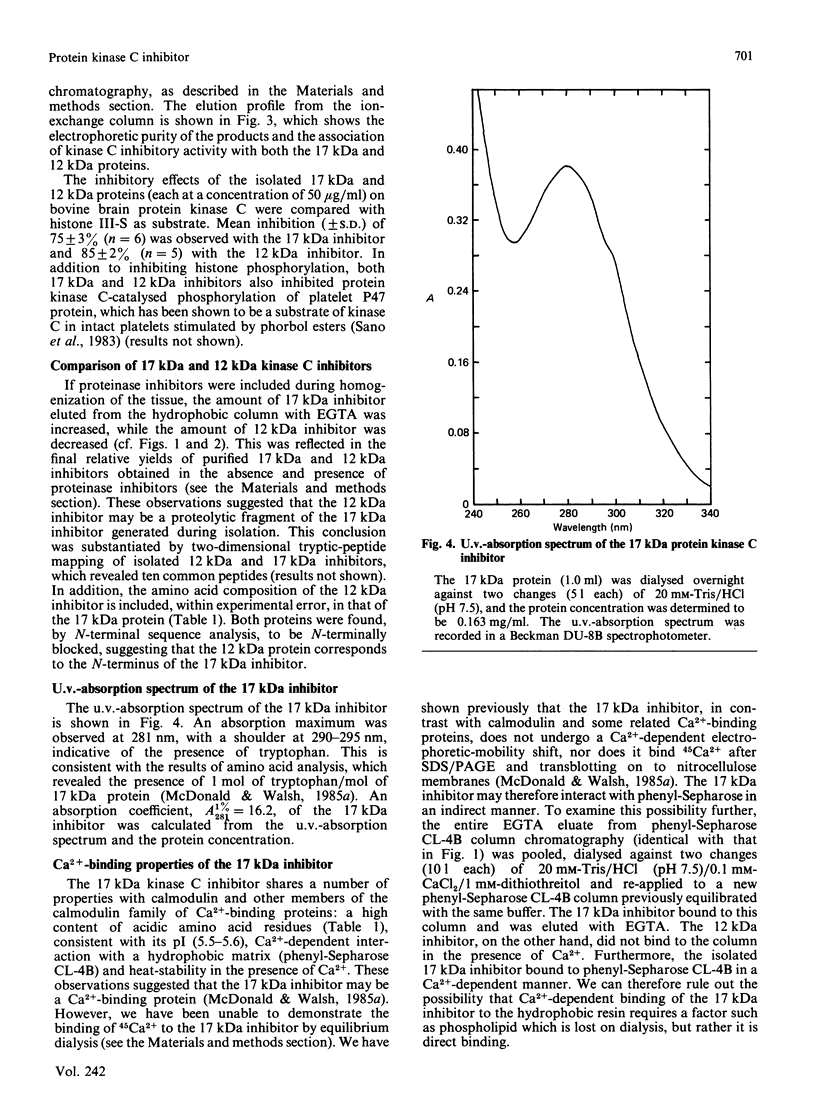
Ca2+-dependent hydrophobic-interaction chromatography is a powerful tool for the identification and isolation of a variety of Ca2+-binding proteins which expose a hydrophobic site(s) in the presence of Ca2+ [Gopalakrishna & Anderson (1982) Biochem. Biophys. Res. Commun. 104, 830-836; Walsh, Valentine, Ngai, Carruthers & Hollenberg (1984) Biochem. J. 224, 117-127; McDonald & Walsh (1985) Biochem. J. 232, 559-567]. Using this approach, we isolated two potent and specific protein inhibitors of protein kinase C, of 17 kDa [McDonald & Walsh (1985) Biochem. J. 232, 559-567] and 12 kDa [McDonald & Walsh (1986) Biochem. Soc. Trans. 14, 585-586]. Although these inhibitors were purified by Ca2+-dependent hydrophobic-interaction chromatography and exhibit properties similar to those of calmodulin and related Ca2+-binding proteins, we were unable to demonstrate high-affinity Ca2+ binding to these inhibitors, using equilibrium dialysis. Protein kinase C exhibited half-maximal activity at 0.6 microM-Ca2+ in the presence of phospholipid and diacylglycerol, and complete inhibition by both inhibitors was observed over the range of Ca2+ concentrations examined (10 nM-10 microM). These observations suggest that the inhibitory action of these proteins does not require Ca2+. The inclusion of proteinase inhibitors during isolation of the kinase C inhibitors, as well as two-dimensional peptide mapping and amino acid analysis of the isolated proteins, suggested that the 12 kDa inhibitor is a proteolytic fragment of the 17 kDa protein which is generated during purification. Antibodies raised in rabbits against the bovine brain 17 kDa inhibitor were shown to be specific by Western immunoblotting and the competitive enzyme-linked immunosorbent assay method and were used to study the tissue and species distribution of this protein. The inhibitor was found to be present in several bovine, murine, avian and human tissues, consistent with a role in the regulation of a variety of physiological functions involving the widely distributed protein kinase C.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berridge M. J. Inositol trisphosphate and diacylglycerol as second messengers. Biochem J. 1984 Jun 1;220(2):345–360. doi: 10.1042/bj2200345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge M. J., Irvine R. F. Inositol trisphosphate, a novel second messenger in cellular signal transduction. Nature. 1984 Nov 22;312(5992):315–321. doi: 10.1038/312315a0. [DOI] [PubMed] [Google Scholar]
- Brock T. A., Rittenhouse S. E., Powers C. W., Ekstein L. S., Gimbrone M. A., Jr, Alexander R. W. Phorbol ester and 1-oleoyl-2-acetylglycerol inhibit angiotensin activation of phospholipase C in cultured vascular smooth muscle cells. J Biol Chem. 1985 Nov 15;260(26):14158–14162. [PubMed] [Google Scholar]
- Cooper R. H., Coll K. E., Williamson J. R. Differential effects of phorbol ester on phenylephrine and vasopressin-induced Ca2+ mobilization in isolated hepatocytes. J Biol Chem. 1985 Mar 25;260(6):3281–3288. [PubMed] [Google Scholar]
- Corvera S., Schwarz K. R., Graham R. M., García-Sáinz J. A. Phorbol esters inhibit alpha 1-adrenergic effects and decrease the affinity of liver cell alpha 1-adrenergic receptors for (-)-epinephrine. J Biol Chem. 1986 Jan 15;261(2):520–526. [PubMed] [Google Scholar]
- Engvall E. Enzyme immunoassay ELISA and EMIT. Methods Enzymol. 1980;70(A):419–439. doi: 10.1016/s0076-6879(80)70067-8. [DOI] [PubMed] [Google Scholar]
- Fabiato A. Myoplasmic free calcium concentration reached during the twitch of an intact isolated cardiac cell and during calcium-induced release of calcium from the sarcoplasmic reticulum of a skinned cardiac cell from the adult rat or rabbit ventricle. J Gen Physiol. 1981 Nov;78(5):457–497. doi: 10.1085/jgp.78.5.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gispen W. H. Phosphoprotein B-50 and phosphoinositides in brain synaptic plasma membranes: a possible feedback relationship. Biochem Soc Trans. 1986 Feb;14(1):163–165. doi: 10.1042/bst0140163. [DOI] [PubMed] [Google Scholar]
- Gopalakrishna R., Anderson W. B. Ca2+-induced hydrophobic site on calmodulin: application for purification of calmodulin by phenyl-Sepharose affinity chromatography. Biochem Biophys Res Commun. 1982 Jan 29;104(2):830–836. doi: 10.1016/0006-291x(82)90712-4. [DOI] [PubMed] [Google Scholar]
- Hidaka H., Inagaki M., Kawamoto S., Sasaki Y. Isoquinolinesulfonamides, novel and potent inhibitors of cyclic nucleotide dependent protein kinase and protein kinase C. Biochemistry. 1984 Oct 9;23(21):5036–5041. doi: 10.1021/bi00316a032. [DOI] [PubMed] [Google Scholar]
- Hirasawa K., Nishizuka Y. Phosphatidylinositol turnover in receptor mechanism and signal transduction. Annu Rev Pharmacol Toxicol. 1985;25:147–170. doi: 10.1146/annurev.pa.25.040185.001051. [DOI] [PubMed] [Google Scholar]
- Imaoka T., Lynham J. A., Haslam R. J. Purification and characterization of the 47,000-dalton protein phosphorylated during degranulation of human platelets. J Biol Chem. 1983 Sep 25;258(18):11404–11414. [PubMed] [Google Scholar]
- Inoue M., Kishimoto A., Takai Y., Nishizuka Y. Studies on a cyclic nucleotide-independent protein kinase and its proenzyme in mammalian tissues. II. Proenzyme and its activation by calcium-dependent protease from rat brain. J Biol Chem. 1977 Nov 10;252(21):7610–7616. [PubMed] [Google Scholar]
- Kikkawa U., Takai Y., Minakuchi R., Inohara S., Nishizuka Y. Calcium-activated, phospholipid-dependent protein kinase from rat brain. Subcellular distribution, purification, and properties. J Biol Chem. 1982 Nov 25;257(22):13341–13348. [PubMed] [Google Scholar]
- Klee C. B. Conformational transition accompanying the binding of Ca2+ to the protein activator of 3',5'-cyclic adenosine monophosphate phosphodiesterase. Biochemistry. 1977 Mar 8;16(5):1017–1024. doi: 10.1021/bi00624a033. [DOI] [PubMed] [Google Scholar]
- Kuo J. F., Andersson R. G., Wise B. C., Mackerlova L., Salomonsson I., Brackett N. L., Katoh N., Shoji M., Wrenn R. W. Calcium-dependent protein kinase: widespread occurrence in various tissues and phyla of the animal kingdom and comparison of effects of phospholipid, calmodulin, and trifluoperazine. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7039–7043. doi: 10.1073/pnas.77.12.7039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lambin P. Reliability of molecular weight determination of proteins by polyacrylamide gradient gel electrophoresis in the presence of sodium dodecyl sulfate. Anal Biochem. 1978 Mar;85(1):114–125. doi: 10.1016/0003-2697(78)90281-6. [DOI] [PubMed] [Google Scholar]
- Leeb-Lundberg L. M., Cotecchia S., Lomasney J. W., DeBernardis J. F., Lefkowitz R. J., Caron M. G. Phorbol esters promote alpha 1-adrenergic receptor phosphorylation and receptor uncoupling from inositol phospholipid metabolism. Proc Natl Acad Sci U S A. 1985 Sep;82(17):5651–5655. doi: 10.1073/pnas.82.17.5651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim M. S., Sutherland C., Walsh M. P. Phosphorylation of bovine cardiac C-protein by protein kinase C. Biochem Biophys Res Commun. 1985 Nov 15;132(3):1187–1195. doi: 10.1016/0006-291x(85)91932-1. [DOI] [PubMed] [Google Scholar]
- May W. S., Jr, Sahyoun N., Wolf M., Cuatrecasas P. Role of intracellular calcium mobilization in the regulation of protein kinase C-mediated membrane processes. Nature. 1985 Oct 10;317(6037):549–551. doi: 10.1038/317549a0. [DOI] [PubMed] [Google Scholar]
- McDonald J. R., Walsh M. P. Ca2+-binding proteins from bovine brain including a potent inhibitor of protein kinase C. Biochem J. 1985 Dec 1;232(2):559–567. doi: 10.1042/bj2320559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald J. R., Walsh M. P. Inhibition of the Ca2+- and phospholipid-dependent protein kinase by a novel Mr 17,000 Ca2+-binding protein. Biochem Biophys Res Commun. 1985 Jun 14;129(2):603–610. doi: 10.1016/0006-291x(85)90194-9. [DOI] [PubMed] [Google Scholar]
- McDonald J. R., Walsh M. P., McCubbin W. D., Oikawa K., Kay C. M. Physicochemical properties of a novel Mr-21 000 Ca2+-binding protein of bovine brain. Biochem J. 1985 Dec 1;232(2):569–575. doi: 10.1042/bj2320569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minakuchi R., Takai Y., Yu B., Nishizuka Y. Widespread occurrence of calcium-activated, phospholipid-dependent protein kinase in mammalian tissues. J Biochem. 1981 May;89(5):1651–1654. doi: 10.1093/oxfordjournals.jbchem.a133362. [DOI] [PubMed] [Google Scholar]
- Naccache P. H., Molski T. F., Borgeat P., White J. R., Sha'afi R. I. Phorbol esters inhibit the fMet-Leu-Phe- and leukotriene B4-stimulated calcium mobilization and enzyme secretion in rabbit neutrophils. J Biol Chem. 1985 Feb 25;260(4):2125–2131. [PubMed] [Google Scholar]
- Ngai P. K., Walsh M. P. Detection of caldesmon in muscle and non-muscle tissues of the chicken using polyclonal antibodies. Biochem Biophys Res Commun. 1985 Mar 15;127(2):533–539. doi: 10.1016/s0006-291x(85)80192-3. [DOI] [PubMed] [Google Scholar]
- Nishizuka Y. The role of protein kinase C in cell surface signal transduction and tumour promotion. Nature. 1984 Apr 19;308(5961):693–698. doi: 10.1038/308693a0. [DOI] [PubMed] [Google Scholar]
- Orellana S. A., Solski P. A., Brown J. H. Phorbol ester inhibits phosphoinositide hydrolysis and calcium mobilization in cultured astrocytoma cells. J Biol Chem. 1985 May 10;260(9):5236–5239. [PubMed] [Google Scholar]
- Sano K., Takai Y., Yamanishi J., Nishizuka Y. A role of calcium-activated phospholipid-dependent protein kinase in human platelet activation. Comparison of thrombin and collagen actions. J Biol Chem. 1983 Feb 10;258(3):2010–2013. [PubMed] [Google Scholar]
- Simpson R. J., Neuberger M. R., Liu T. Y. Complete amino acid analysis of proteins from a single hydrolysate. J Biol Chem. 1976 Apr 10;251(7):1936–1940. [PubMed] [Google Scholar]
- Singh T. J., Akatsuka A., Huang K. P., Sharma R. K., Tam S. W., Wang J. H. A multifunctional cyclic nucleotide- and Ca2+-independent protein kinase from rabbit skeletal muscle. Biochem Biophys Res Commun. 1982 Jul 30;107(2):676–683. doi: 10.1016/0006-291x(82)91544-3. [DOI] [PubMed] [Google Scholar]
- Spector T. Refinement of the coomassie blue method of protein quantitation. A simple and linear spectrophotometric assay for less than or equal to 0.5 to 50 microgram of protein. Anal Biochem. 1978 May;86(1):142–146. doi: 10.1016/0003-2697(78)90327-5. [DOI] [PubMed] [Google Scholar]
- Su H. D., Kemp B. E., Turner R. S., Kuo J. F. Synthetic myelin basic protein peptide analogs are specific inhibitors of phospholipid/calcium-dependent protein kinase (protein kinase C). Biochem Biophys Res Commun. 1986 Jan 14;134(1):78–84. doi: 10.1016/0006-291x(86)90529-2. [DOI] [PubMed] [Google Scholar]
- Südhof T. C., Ebbecke M., Walker J. H., Fritsche U., Boustead C. Isolation of mammalian calelectrins: a new class of ubiquitous Ca2+-regulated proteins. Biochemistry. 1984 Mar 13;23(6):1103–1109. doi: 10.1021/bi00301a010. [DOI] [PubMed] [Google Scholar]
- Thomopoulos P., Testa U., Gourdin M. F., Hervy C., Titeux M., Vainchenker W. Inhibition of insulin receptor binding by phorbol esters. Eur J Biochem. 1982 Dec 15;129(2):389–393. doi: 10.1111/j.1432-1033.1982.tb07062.x. [DOI] [PubMed] [Google Scholar]
- Waisman D. M., Muranyi J., Ahmed M. Identification of a novel calcium binding protein from bovine brain. FEBS Lett. 1983 Nov 28;164(1):80–84. doi: 10.1016/0014-5793(83)80023-4. [DOI] [PubMed] [Google Scholar]
- Waisman D. M., Salimath B. P., Anderson M. J. Isolation and characterization of CAB-63, a novel calcium-binding protein. J Biol Chem. 1985 Feb 10;260(3):1652–1660. [PubMed] [Google Scholar]
- Walsh D. A., Ashby C. D., Gonzalez C., Calkins D., Fischer E. H. Krebs EG: Purification and characterization of a protein inhibitor of adenosine 3',5'-monophosphate-dependent protein kinases. J Biol Chem. 1971 Apr 10;246(7):1977–1985. [PubMed] [Google Scholar]
- Walsh M. P., Hinkins S., Dabrowska R., Hartshorne D. J. Smooth muscle myosin light chain kinase. Methods Enzymol. 1983;99:279–288. doi: 10.1016/0076-6879(83)99063-8. [DOI] [PubMed] [Google Scholar]
- Walsh M. P., Valentine K. A., Ngai P. K., Carruthers C. A., Hollenberg M. D. Ca2+-dependent hydrophobic-interaction chromatography. Isolation of a novel Ca2+-binding protein and protein kinase C from bovine brain. Biochem J. 1984 Nov 15;224(1):117–127. doi: 10.1042/bj2240117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson S. P., Lapetina E. G. 1,2-Diacylglycerol and phorbol ester inhibit agonist-induced formation of inositol phosphates in human platelets: possible implications for negative feedback regulation of inositol phospholipid hydrolysis. Proc Natl Acad Sci U S A. 1985 May;82(9):2623–2626. doi: 10.1073/pnas.82.9.2623. [DOI] [PMC free article] [PubMed] [Google Scholar]