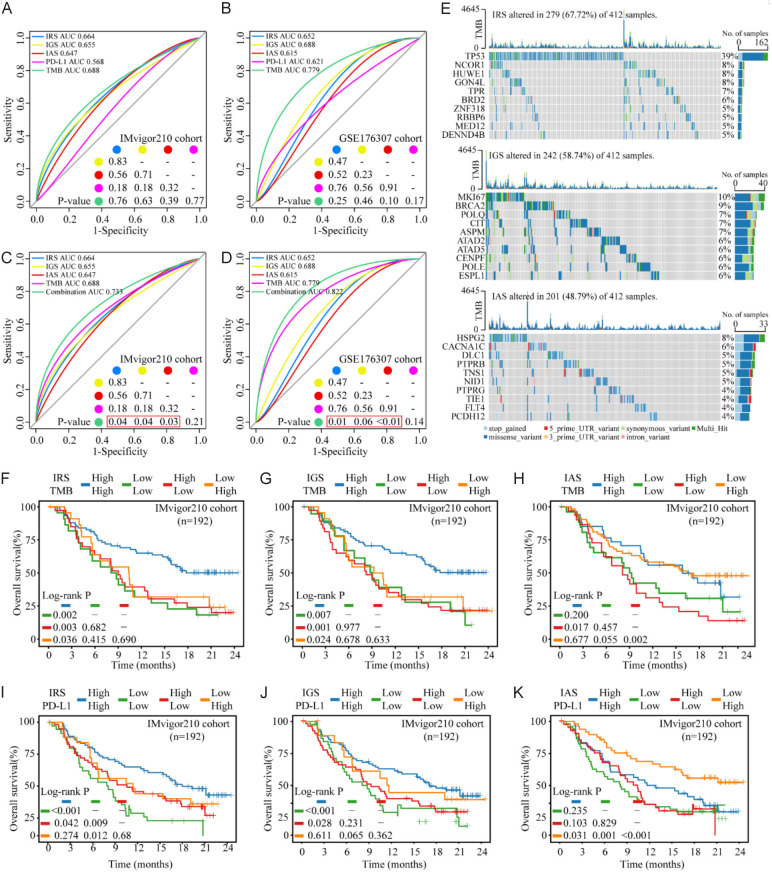
Figure 4.
ICBT signatures and TMB or PD-L1 panel predicts response and benefit of ICBT in BLCA. (A and B) Predictive power of TMB, PD-L1, and ICBT signatures used independently for response of ICBT in the IMvigor210 cohort (A) and GSE176307 cohort (B). (C and D) The combination of ICBT signatures and TMB could significantly improve the predictive ability of ICBT signatures for the response of ICBT in the IMvigor210 cohort (C) and GSE176307 cohort (D). (E) The landscape of the top 10 most frequently mutated genes for each ICBT signature. (F-H) Kaplan-Meier survival curves of IRS/IGS/IAS and TMB panel for OS of patients with ICBT from IMvigor210 cohort. (I-K) Kaplan-Meier survival curves of IRS/IGS/IAS and PD-L1 panel for OS of patients with ICBT from IMvigor210 cohort. ICBT, immune checkpoint blockade therapy; TMB, tumor mutation burden; BLCA, bladder urothelial carcinoma; PD-L1, programmed cell death ligand-1; IRS, ICBT RNA regulatory signature; IGS, ICBT genomic stability signature; IAS, ICBT angiogenesis signature; OS, overall survival.