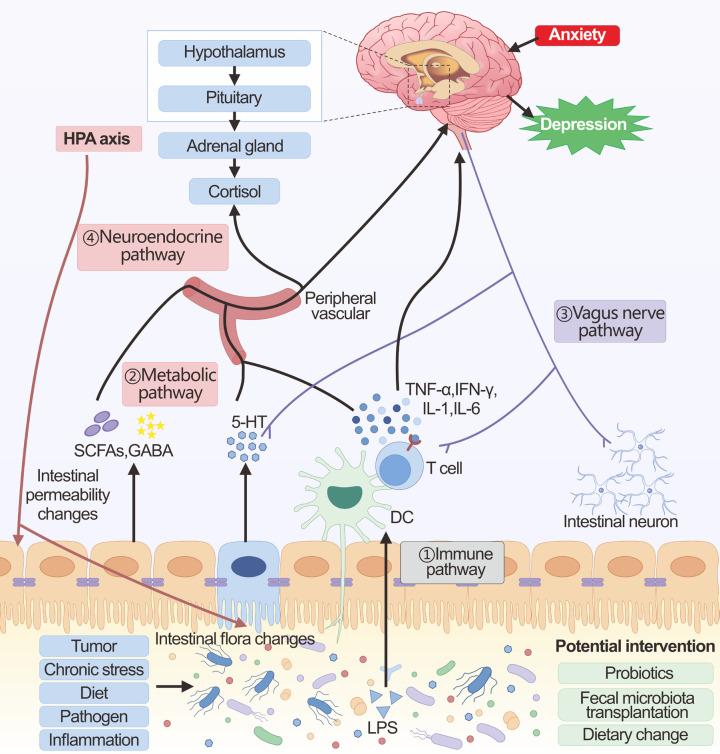
Figure 2.
The brain-gut axis in cancer-related depression. The main communication pathways between the brain-gut axis in the context of gut microbiota homeostasis include immune, metabolic, vagus nerve, and neuroendocrine pathways. ① Immune pathway: Alteration of the gut microbiota and translocation of LPS activates the immune system. The induced pro-inflammatory cytokines can then affect brain function either directly through the blood-brain barrier or using the vagal pathway. ② Metabolic pathway: The intestinal microbiota produces a large number of metabolites (SCFAs, GABA, 5-HT, etc.), which can enter the brain through the peripheral circulation, affecting the glial cells and neural plasticity. In addition, these metabolites also affect brain function by affecting both the vagus nerve and the HPA axis. ③ Vagus nerve pathway: Changes in the gut microbiota, microbial products, and microbiota-dependent immune mediators can directly interact with intestinal neurons and the vagus nerve, which can be transmitted via sensory circuits to brain regions involved in cognition and mood. ④ Neuroendocrine pathway: The neuroendocrine pathway is mainly regulated through the HPA axis. The HPA axis can be activated by chronic stress, as well as by the immune-inflammatory response induced by microbial alterations in the gut and by metabolites. The large amounts of cortisol released by over-activation of the HPA axis cause shifts in the composition of the gut microbiota as well as changes in the permeability of the gut barrier.