Figure 4.
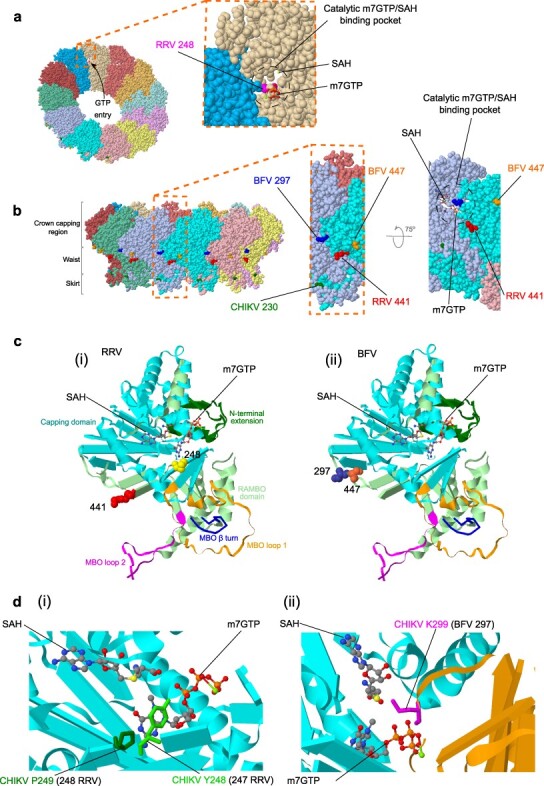
Mapping of convergent RRV/BFV nsP1 mutations to CHIKV cryo-EM structures. Cryo-EM crystal structure of the CHIKV nsP1 oligomer complex based on PDB code 7FGG (PDB DOI: 10.2210/pdb7FGG/pdb; Zhang et al. 2022) with 7-methylguanosine 5ʹ-triphosphate (m7GTP) and S-adenosyl homocysteine [SAH, byproduct produced during methylation of GTP requiring S-adenosyl-l-methionine (SAM)-dependent methyltransferase (MTase) and m7guanosine-5ʹ-triphosphate (GTP) transferase (GTase)] shown as sticks. (A) The 12 nsP1 monomers forming the ring structure are individually colored and the entrance of GTP into the catalytic m7GTP/SAH binding pocket is shown by an arrow. The inset shows how the catalytic pocket, which is involved during RNA capping, is formed from adjacent nsP1 molecules and the predicted position of mutated RRV site 248 is highlighted. (B) Lateral view of the nsP1 complex represented in (A) showing the three recently described regions (crown, waist, and skirt; Jones et al. 2021) and predicted positioning of RRV and BFV nsP1 mutations. The location of CHIKV site 230 implicated with site 299 (BFV site 297) in mutations, which confer resistance to the antiviral compound FHA (Kovacikova et al. 2021) is also highlighted. The inset further shows the relative proximity of the mutated RRV site 441 and BFV sites 297 and 447 within the nsP1 complex waist region involved with host cell membrane binding. Upward rotation of this magnified view allows visualization of the catalytic m7GTP/SAH binding pocket. (C) The nsP1 monomer structure showing the mapped positioning of (i) RRV and (ii) BFV nsP1 mutations relative to m7GTP and SAH bound molecules. (D) Close-up views showing key CHIKV residues (also represented as sticks) involved with m7GTP/SAH binding. The corresponding mutation site numbers for (i) RRV and (ii) BFV are shown in brackets.
