Figure 2.
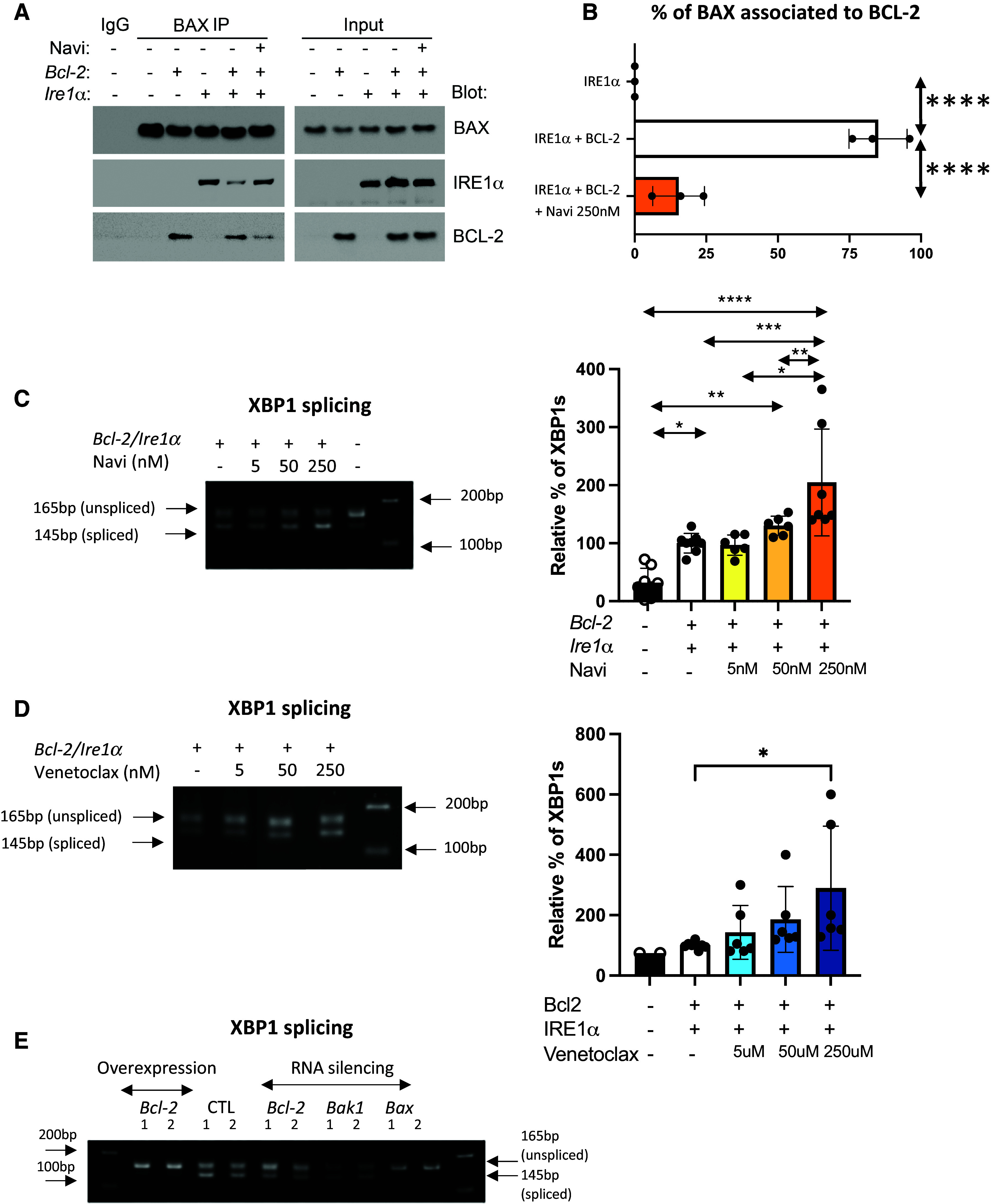
BCL-2 regulates IRE1α activation by sequestering BAX. (A) Coimmunoprecipitation of BAX with BCL-2 or IRE1α from human embryonic kidney 293 (HEK-293) cells cotransfected with BCL-2 and IRE1α plasmids followed by treatment with navitoclax (250 nM). BAX was immunoprecipitated (BAX IP), and BCL-2 and IRE1α were detected by western blot. n = 3 biological replicates. (B) Quantification of the intensity of bands, expressed as percentage of BAX associated with BCL-2. (C and D) Representative ethidium bromide–stained agarose gel of Xbp1 cDNA amplicons after transfection of Bcl-2 and pcDNA3 and Ire1α plasmid in HEK-293 cells followed by treatment with (C) navitoclax (5, 50, and 250 nM) or (D) venetoclax (5, 50, and 250 nM) for 24 hours. n = 6 (2 technical replicates in 3 biological replicates). Bar graphs indicate the percentage of spliced-form amplicons over spliced plus unspliced amplicons in samples transfected with Bcl-2 and pcDNA3 and Ire1α plasmid (control). The percentage of Xbp1 splicing in each control sample per replicate is the reference and equals 100. n = 6 (2 technical replicates in 3 biological replicates). (E) Representative ethidium bromide–stained agarose gel of Xbp1 cDNA amplicons after transfection of siRNA targeting Bcl-2, Bax, and Bak-1 in HEK-293 cells. n = 6 (2 technical replicates in 3 biological replicates). All data represent mean ± SD. *P < 0.05, **P < 0.01, ***P < 0.001, and ****P < 0.0001, as determined by one-way ANOVA followed by Tukey multiple comparison test in (B–D).
