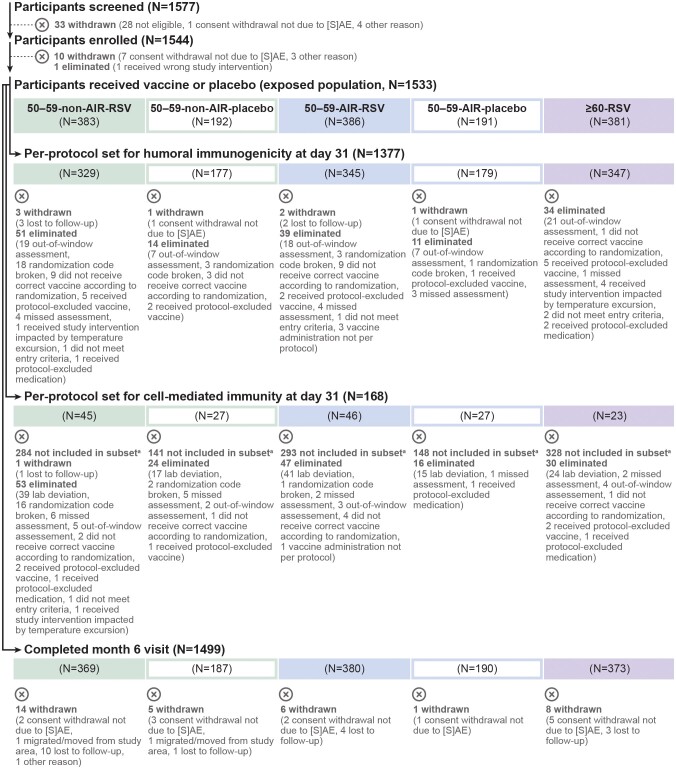
Figure 3.
Flow of participants. For eliminations from the per-protocol sets for immunogenicity, multiple reasons could apply for 1 participant; all reasons are listed in the figure. Abbreviations: (S)AE, (serious) adverse event; 50–59-AIR-placebo, group of 50–59-year-old participants at increased risk for respiratory syncytial virus (RSV) disease who received placebo; 50–59-AIR-RSV, group of 50–59-year-old participants at increased risk for RSV disease who received RSV prefusion F protein–based vaccine (RSVPreF3 OA); 50–59-non-AIR-placebo, group of 50–59-year-old participants without increased risk for RSV disease who received placebo; 50–59-non-AIR-RSV, group of 50–59-year-old participants without increased risk for RSV disease who received RSVPreF3 OA; ≥60-RSV, group of ≥60-year-old participants who received RSVPreF3 OA. aParticipants in the cell-mediated immunity subset were recruited from a selected number of countries and centers (Supplementary Methods). In total, 339 participants were included in the exposed set of the cell-mediated immunity subset.