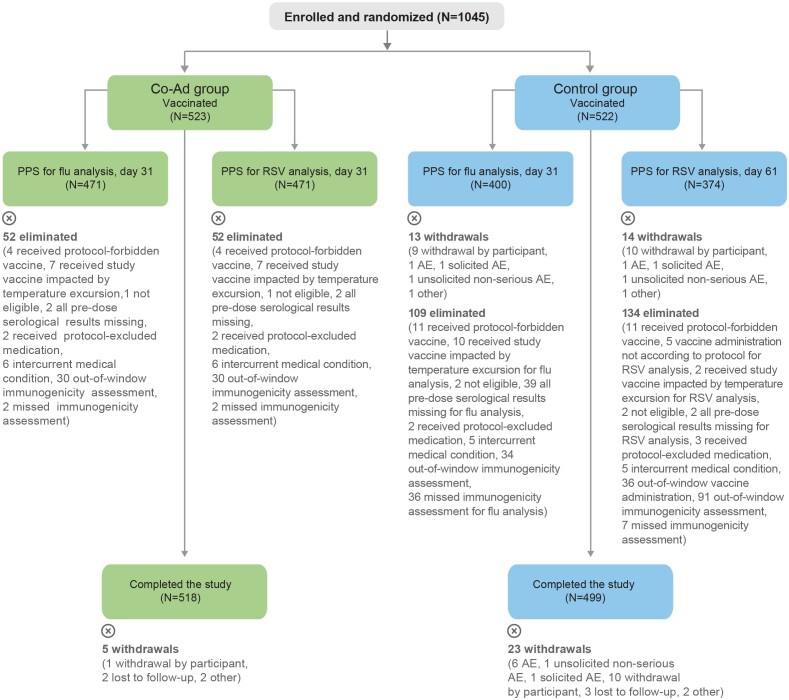
Figure 2.
Flow of participants. For eliminations from the PPS, multiple reasons could apply for 1 participant; all reasons are listed in the figure. Co-Ad, group of participants who received RSVPreF3 OA and FLU-aQIV concomitantly on day 1. Control, group of participants who received FLU-aQIV on day 1 and RSVPreF3 OA on day 31; N, number of participants in the indicated analysis set and group. Abbreviations: AE, adverse event; FLU-aQIV, adjuvanted inactivated seasonal quadrivalent influenza vaccine; PPS, per-protocol sets for immunogenicity; RSVPreF3 OA, respiratory syncytial virus (RSV) prefusion F protein-based vaccine.