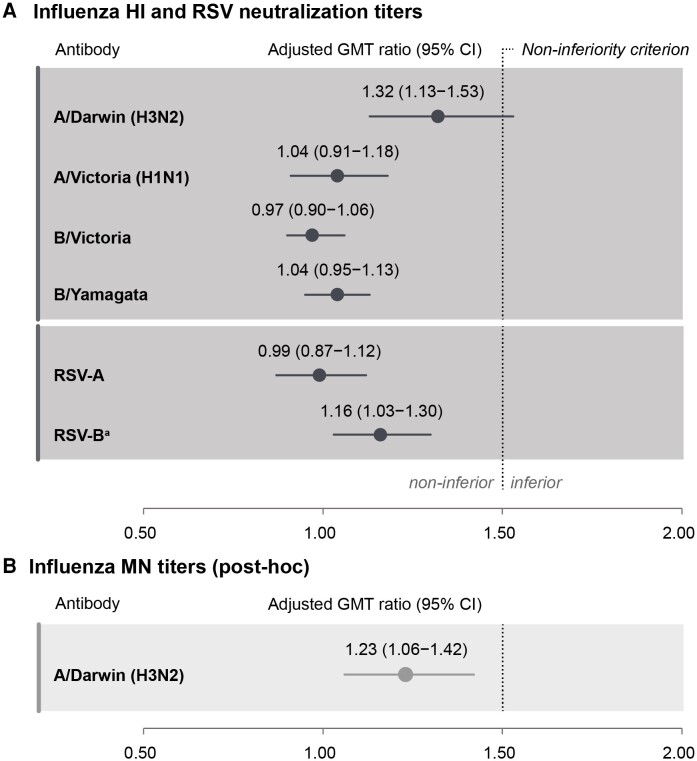
Figure 3.
Non-inferiority of FLU-aQIV and RSVPreF3 OA co-administration versus sequential administration, evaluated by adjusted GMT ratios for influenza HI and RSV neutralizing titers A, as well as influenza MN titers for A/Darwin (H3N2) B, (per-protocol sets for flu and RSV analyses). CIs are depicted as error bars. Success criterion for non-inferiority: upper limits of 2-sided 95% CIs for adjusted geometric mean titer (GMT) ratios (Control/Co-Ad) for each FLU-aQIV strain and for RSV-A and RSV-B were ≤1.50 at 1 m post-vaccination. Abbreviations: CI, confidence interval; FLU-aQIV, adjuvanted inactivated seasonal quadrivalent influenza vaccine; HI, hemagglutination inhibition; MN, microneutralization; RSVPreF3 OA, respiratory syncytial virus (RSV) prefusion F protein-based vaccine. aAs the non-inferiority analysis was performed sequentially, and the success criterion was not met for A/Darwin (H3N2), non-inferiority for RSV-B neutralization titers was no longer a confirmatory endpoint and was evaluated descriptively.