Abstract
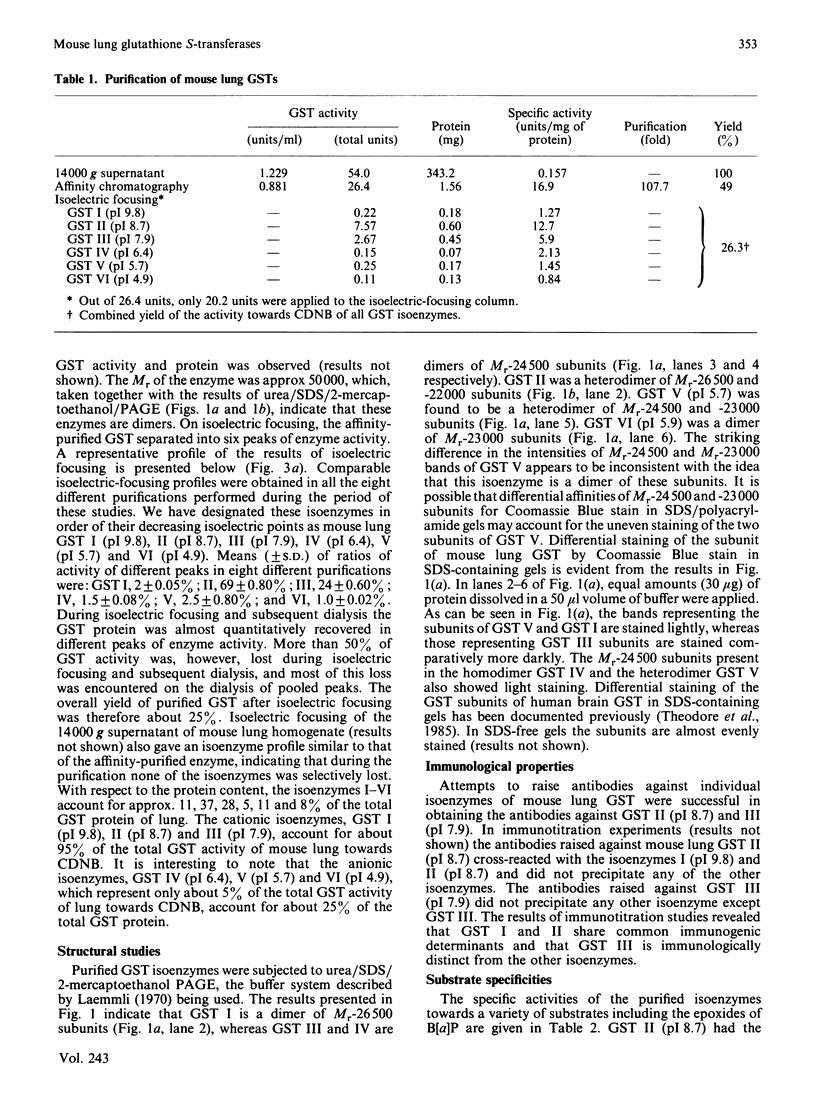
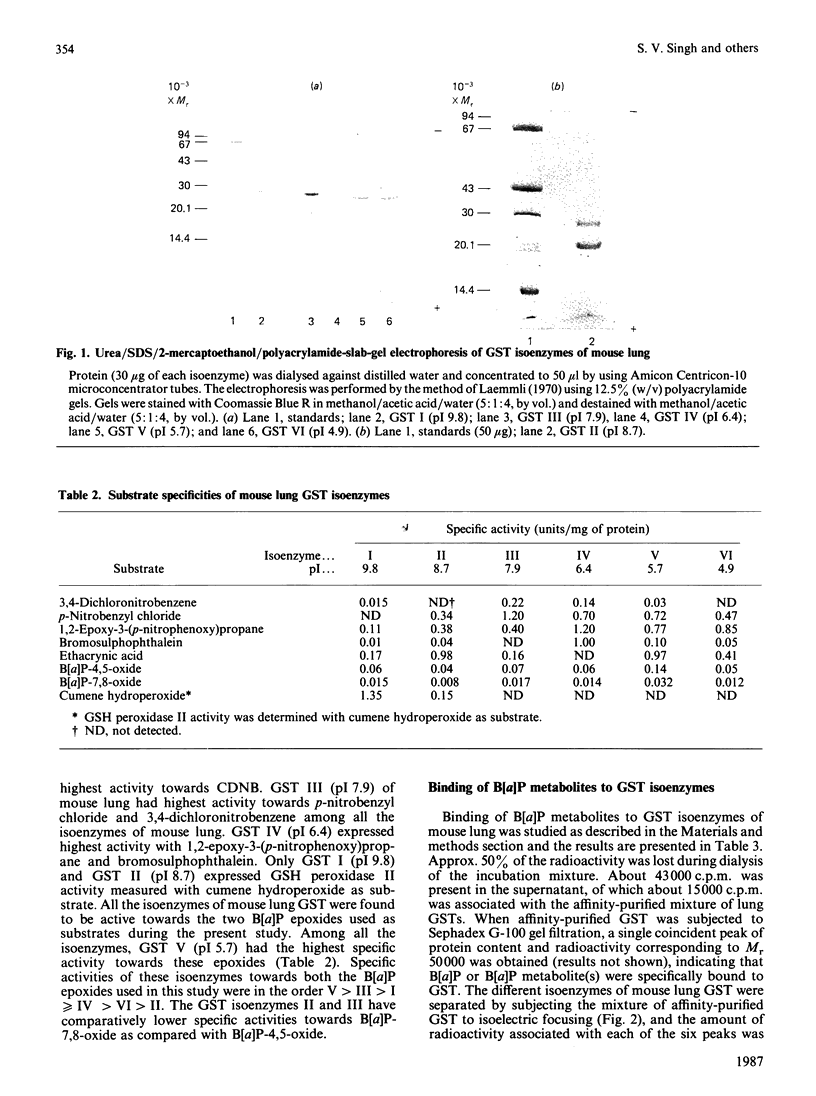
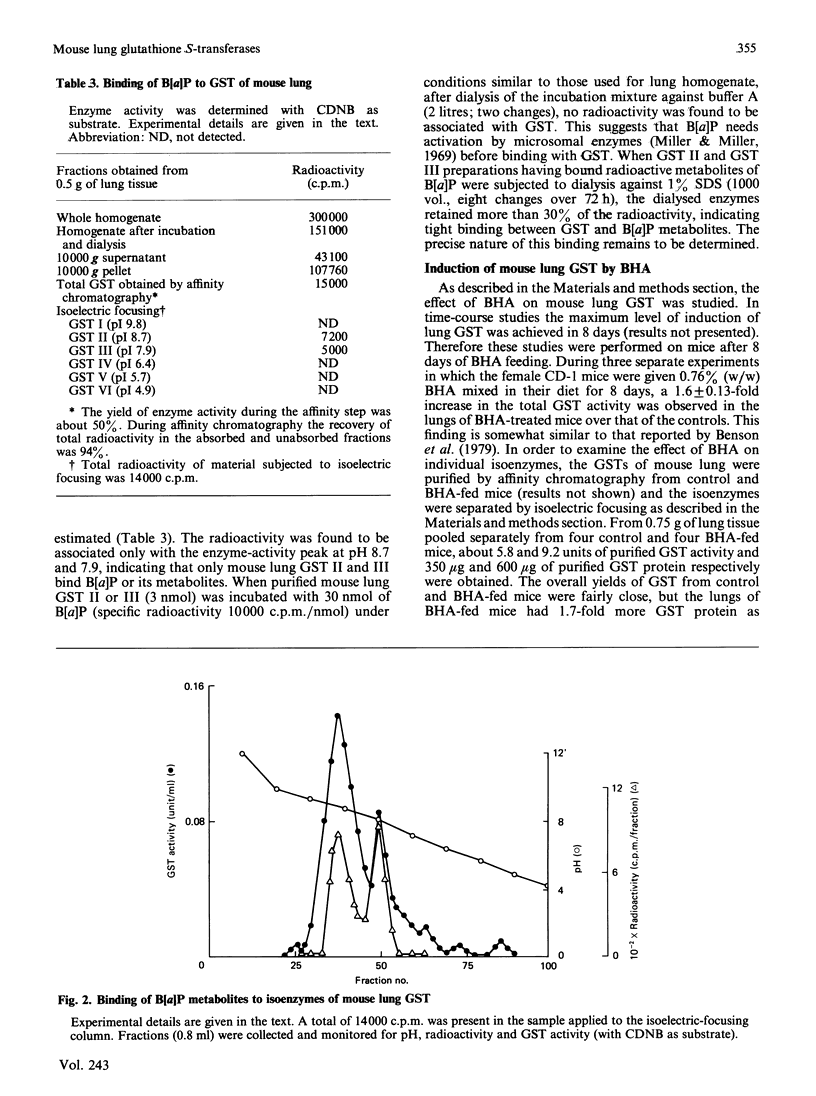
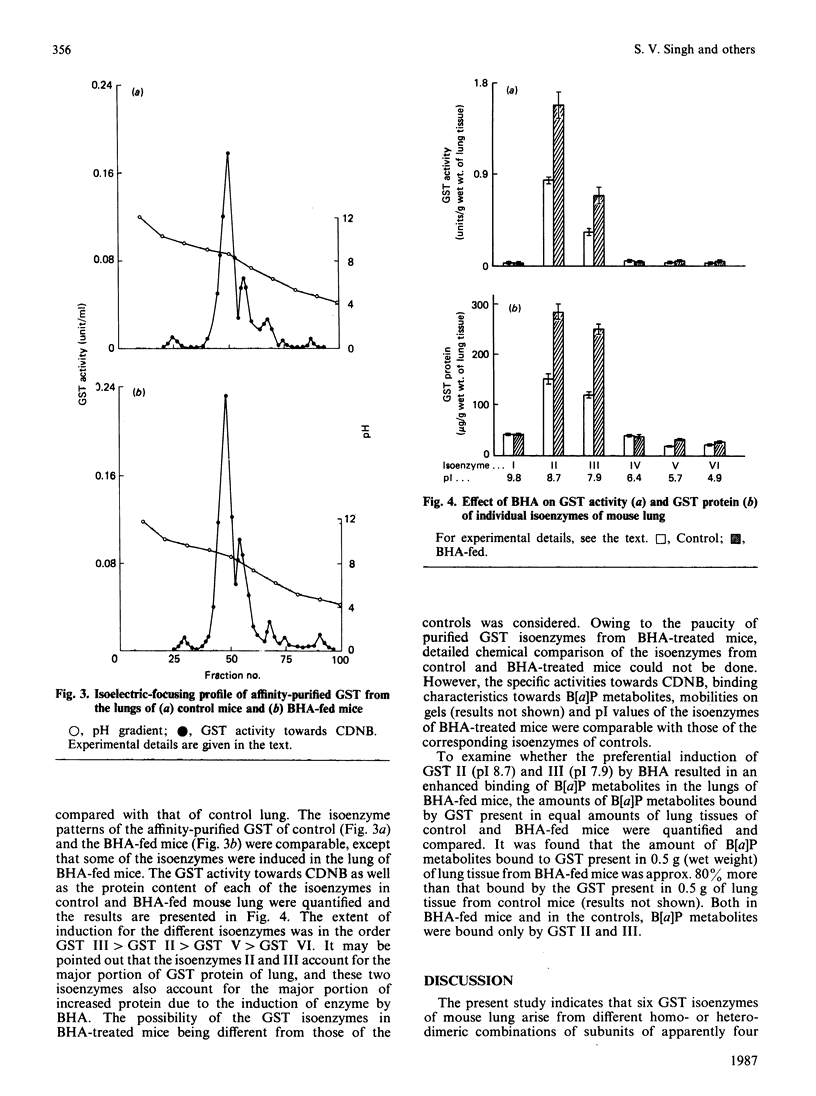
Six isoenzymes of glutathione S-transferase (GST) present in mouse lung have been purified and characterized. GST I (pI 9.8) is a dimer of Mr-26,500 subunits and GST II is a heterodimer of Mr-26,500 and -22,000 subunits, and GST III (pI 7.9) and IV (pI 6.4) are dimers of Mr-24,500 subunits. GST V (pI 5.7) is a heterodimer of Mr-24,500 and -23,000 subunits, whereas GST VI (pI 4.9) is a dimer of Mr-23,000 subunits. Immunological studies indicate that the Mr-24,500 subunits present in GST III (pI 7.9) are distinct from those present in GST IV (pI 6.4) and V (pI 5.7). Structural and immunological studies provide evidence that at least five distinct types of subunits in their different binary combinations give rise to various GST isoenzymes of mouse lung. These isoenzymes express varying degrees of catalytic activities towards a wide range of electrophilic substrates including benzo[a]pyrene 7,8-oxide and benzo[a]pyrene 4,5-oxide. The dietary antioxidant t-butylated hydroxyanisole (BHA) preferentially induces GST II and III. Also, these two isoenzymes selectively bind benzo[a]pyrene (B[a]P) metabolites, indicating that they play an important physiological role in the detoxification of B[a]P metabolites. The preferential induction of the GST isoenzymes involved in the detoxification of activated B[a]P metabolites indicates that the anti-neoplastic activity of BHA against B[a]P-induced neoplasia in mouse lung [Wattenberg (1973) J. Natl. Cancer Inst. 50, 1541-1544] may be due to the enhanced detoxification of B[a]P metabolites.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Awasthi Y. C., Beutler E., Srivastava S. K. Purification and properties of human erythrocyte glutathione peroxidase. J Biol Chem. 1975 Jul 10;250(13):5144–5149. [PubMed] [Google Scholar]
- Awasthi Y. C., Dao D. D., Saneto R. P. Interrelationship between anionic and cationic forms of glutathione S-transferases of human liver. Biochem J. 1980 Oct 1;191(1):1–10. doi: 10.1042/bj1910001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Awasthi Y. C., Partridge C. A., Dao D. D. Effect of butylated hydroxytoluene on glutathione S-transferase and glutathione peroxidase activities in rat liver. Biochem Pharmacol. 1983 Apr 1;32(7):1197–1200. doi: 10.1016/0006-2952(83)90271-x. [DOI] [PubMed] [Google Scholar]
- Awasthi Y. C., Partridge C. A., Theodore C., Dao D. D. Comparative effect of the induction of the subunits of rat liver glutathione S-transferases by butylated hydroxytoluene. Comp Biochem Physiol C. 1984;78(1):39–41. doi: 10.1016/0742-8413(84)90044-6. [DOI] [PubMed] [Google Scholar]
- Awasthi Y. C., Singh S. V. Subunit structure of human and rat glutathione S-transferases. Comp Biochem Physiol B. 1985;82(1):17–23. doi: 10.1016/0305-0491(85)90121-x. [DOI] [PubMed] [Google Scholar]
- Benson A. M., Batzinger R. P., Ou S. Y., Bueding E., Cha Y. N., Talalay P. Elevation of hepatic glutathione S-transferase activities and protection against mutagenic metabolites of benzo(a)pyrene by dietary antioxidants. Cancer Res. 1978 Dec;38(12):4486–4495. [PubMed] [Google Scholar]
- Benson A. M., Cha Y. N., Bueding E., Heine H. S., Talalay P. Elevation of extrahepatic glutathione S-transferase and epoxide hydratase activities by 2(3)-tert-butyl-4-hydroxyanisole. Cancer Res. 1979 Aug;39(8):2971–2977. [PubMed] [Google Scholar]
- Booth J., Boyland E., Sims P. An enzyme from rat liver catalysing conjugations with glutathione. Biochem J. 1961 Jun;79(3):516–524. doi: 10.1042/bj0790516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Chasseaud L. F. The role of glutathione and glutathione S-transferases in the metabolism of chemical carcinogens and other electrophilic agents. Adv Cancer Res. 1979;29:175–274. doi: 10.1016/s0065-230x(08)60848-9. [DOI] [PubMed] [Google Scholar]
- Habig W. H., Pabst M. J., Jakoby W. B. Glutathione S-transferases. The first enzymatic step in mercapturic acid formation. J Biol Chem. 1974 Nov 25;249(22):7130–7139. [PubMed] [Google Scholar]
- Hayes J. D., Mantle T. J. Anomalous electrophoretic behaviour of the glutathione S-transferase Ya and Yk subunits isolated from man and rodents. A potential pitfall for nomenclature. Biochem J. 1986 Aug 1;237(3):731–740. doi: 10.1042/bj2370731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakoby W. B. The glutathione S-transferases: a group of multifunctional detoxification proteins. Adv Enzymol Relat Areas Mol Biol. 1978;46:383–414. doi: 10.1002/9780470122914.ch6. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lee C. Y., Johnson L., Cox R. H., McKinney J. D., Lee S. M. Mouse liver glutathione S-transferases. Biochemical and immunological characterization. J Biol Chem. 1981 Aug 10;256(15):8110–8116. [PubMed] [Google Scholar]
- Litwack G., Ketterer B., Arias I. M. Ligandin: a hepatic protein which binds steroids, bilirubin, carcinogens and a number of exogenous organic anions. Nature. 1971 Dec 24;234(5330):466–467. doi: 10.1038/234466a0. [DOI] [PubMed] [Google Scholar]
- Miller J. A., Miller E. C. The metabolic activation of carcinogenic aromatic amines and amides. Prog Exp Tumor Res. 1969;11:273–301. doi: 10.1159/000391399. [DOI] [PubMed] [Google Scholar]
- Mukhtar H., Bend J. R. Serum glutathione S-transferases: perinatal development, sex difference, and effect of carbon tetrachloride administration on enzyme activity in the rat. Life Sci. 1977 Nov 1;21(9):1277–1286. doi: 10.1016/0024-3205(77)90008-x. [DOI] [PubMed] [Google Scholar]
- Nakagawa Y., Hiraga K., Suga T. Effects of butylated hydroxytoluene (BHT) on the level of glutathione and the activity of glutathione-S-transferase in rat liver. J Pharmacobiodyn. 1981 Oct;4(10):823–826. doi: 10.1248/bpb1978.4.823. [DOI] [PubMed] [Google Scholar]
- Pantarotto C., Arboix M., Sezzano P., Abbruzzi R. Studies on 5,5-diphenylhydantoin irreversible binding to rat liver microsomal proteins. Biochem Pharmacol. 1982 Apr 15;31(8):1501–1507. doi: 10.1016/0006-2952(82)90372-0. [DOI] [PubMed] [Google Scholar]
- Partridge C. A., Dao D. D., Awasthi Y. C. Glutathione S-transferases of lung: purification and characterization of human lung glutathione S-transferases. Lung. 1984;162(1):27–36. doi: 10.1007/BF02715625. [DOI] [PubMed] [Google Scholar]
- Partridge C. A., Singh S. V., Hong T. D., Theodore C., Dao D. D., Awasthi Y. C. Rat lung glutathione S-transferases: subunit structure and the interrelationship with the liver enzymes. Int J Biochem. 1985;17(3):331–340. doi: 10.1016/0020-711x(85)90208-3. [DOI] [PubMed] [Google Scholar]
- Pearson W. R., Windle J. J., Morrow J. F., Benson A. M., Talalay P. Increased synthesis of glutathione S-transferases in response to anticarcinogenic antioxidants. Cloning and measurement of messenger RNA. J Biol Chem. 1983 Feb 10;258(3):2052–2062. [PubMed] [Google Scholar]
- Simons P. C., Vander Jagt D. L. Purification of glutathione S-transferases from human liver by glutathione-affinity chromatography. Anal Biochem. 1977 Oct;82(2):334–341. doi: 10.1016/0003-2697(77)90169-5. [DOI] [PubMed] [Google Scholar]
- Singh S. V., Partridge C. A., Awasthi Y. C. Rat lung glutathione S-transferases. Evidence for two distinct types of 22000-Mr subunits. Biochem J. 1984 Aug 1;221(3):609–615. doi: 10.1042/bj2210609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh S. V., Srivastava S. K., Awasthi Y. C. Effect of 3,5-di-t-butyl-4-hydroxytoluene (BHT) on glutathione-linked detoxification mechanisms of rat ocular lens. Exp Eye Res. 1985 Sep;41(3):405–413. doi: 10.1016/s0014-4835(85)80031-2. [DOI] [PubMed] [Google Scholar]
- Theodore C., Singh S. V., Hong T. D., Awasthi Y. C. Glutathione S-transferases of human brain. Evidence for two immunologically distinct types of 26500-Mr subunits. Biochem J. 1985 Jan 15;225(2):375–382. doi: 10.1042/bj2250375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tu C. P., Weiss M. J., Li N. Q., Reddy C. C. Tissue-specific expression of the rat glutathione S-transferases. J Biol Chem. 1983 Apr 25;258(8):4659–4662. [PubMed] [Google Scholar]
- Warholm M., Jensson H., Tahir M. K., Mannervik B. Purification and characterization of three distinct glutathione transferases from mouse liver. Biochemistry. 1986 Jul 15;25(14):4119–4125. doi: 10.1021/bi00362a020. [DOI] [PubMed] [Google Scholar]
- Wattenberg L. W. Effects of dietary constituents on the metabolism of chemical carcinogens. Cancer Res. 1975 Nov;35(11 Pt 2):3326–3331. [PubMed] [Google Scholar]
- Wattenberg L. W. Inhibition of carcinogenic and toxic effects of polycyclic hydrocarbons by phenolic antioxidants and ethoxyquin. J Natl Cancer Inst. 1972 May;48(5):1425–1430. [PubMed] [Google Scholar]
- Wattenberg L. W. Inhibition of chemical carcinogen-induced pulmonary neoplasia by butylated hydroxyanisole. J Natl Cancer Inst. 1973 Jun;50(6):1541–1544. doi: 10.1093/jnci/50.6.1541. [DOI] [PubMed] [Google Scholar]



