Abstract
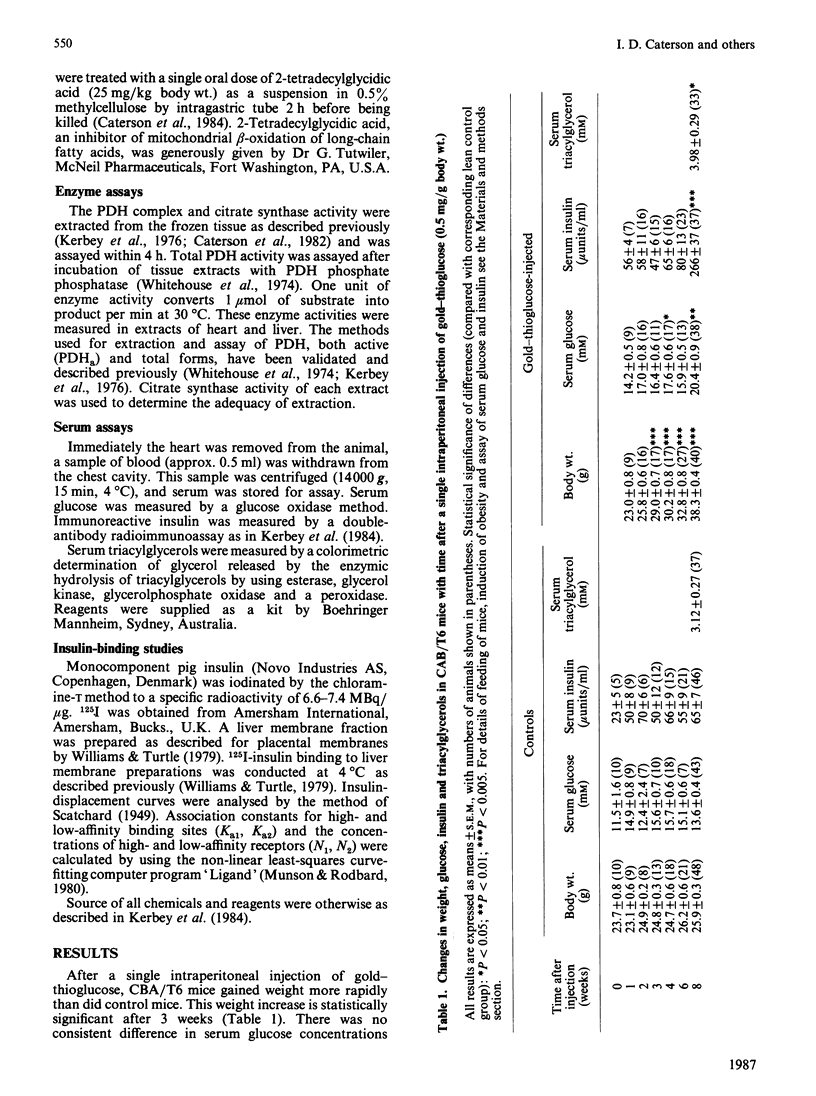
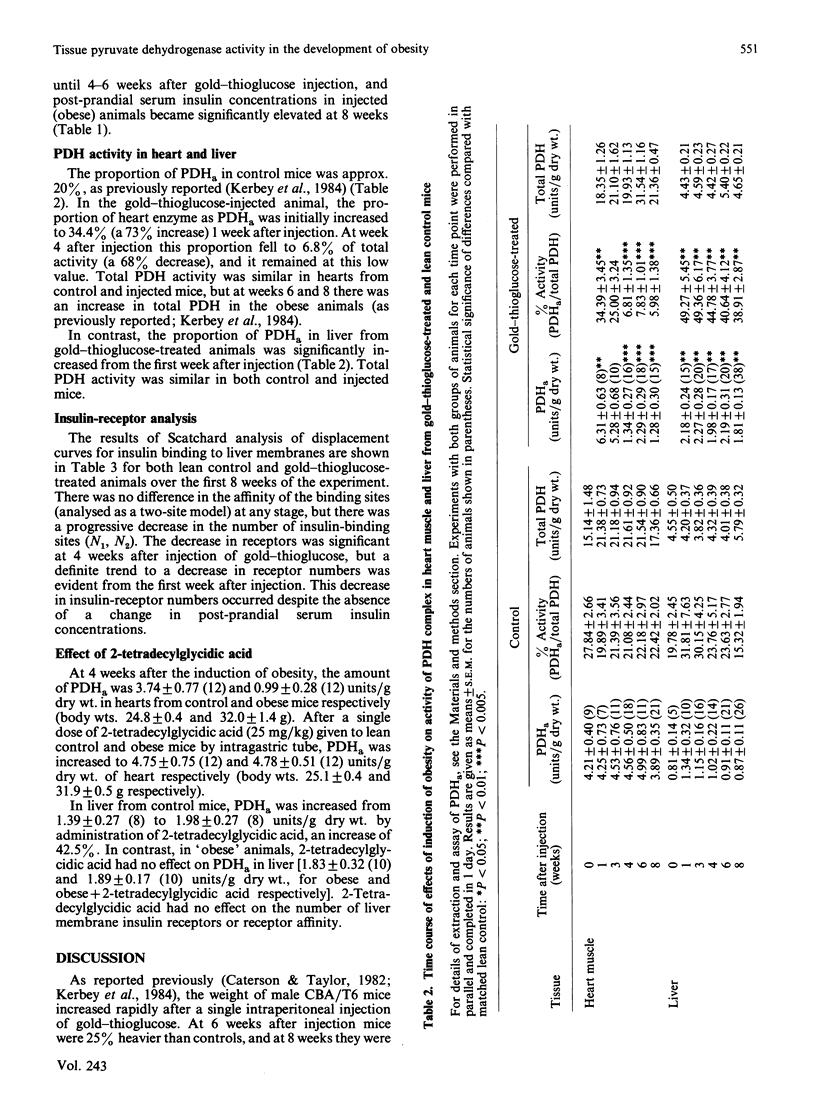
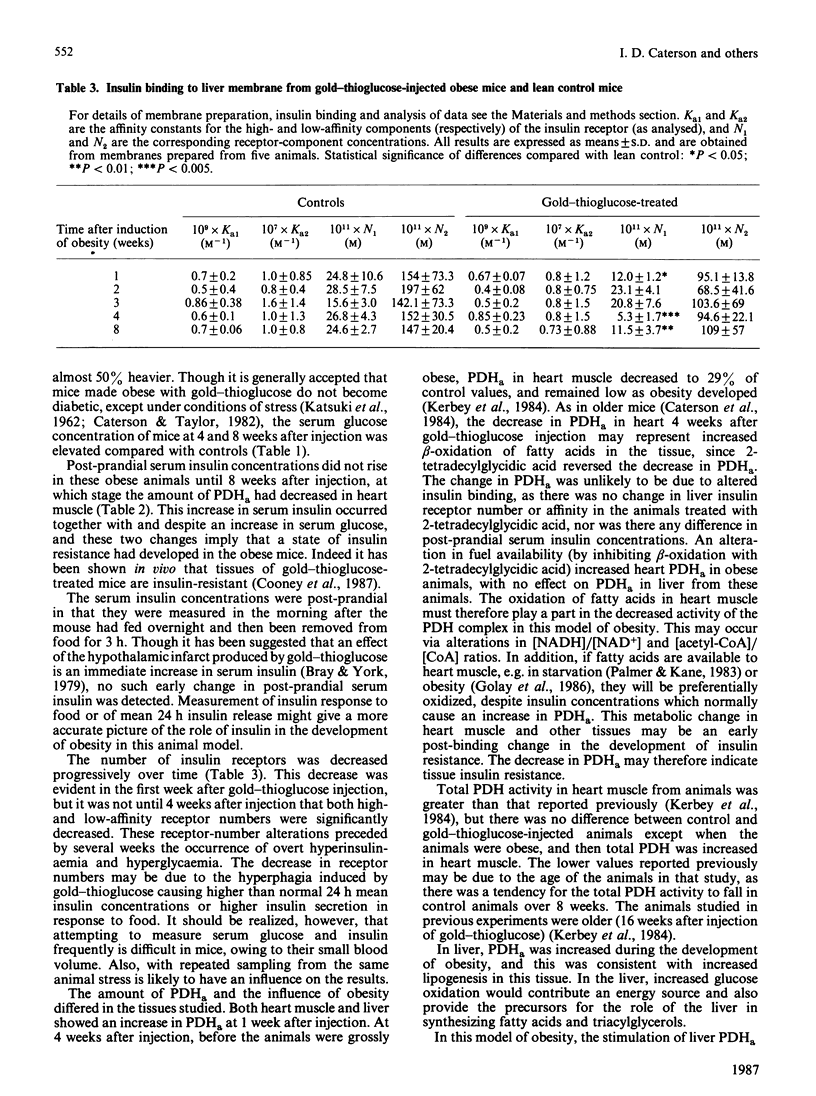
The amount of pyruvate dehydrogenase in the active form (PDHa) was increased 1.7-fold compared with controls in heart muscle of mice 1 week after induction of obesity with a single injection of gold-thioglucose. At 4 weeks post injection, the amount of PDHa was decreased to 32% of control, a value which was observed in later stages of the obesity syndrome. In contrast, liver PDHa was increased and remained at an increased activity during the development of obesity. Despite normal post-prandial serum insulin contents, liver membrane insulin-receptor numbers were decreased 1 week after gold-thioglucose injection, and there was no change in receptor affinity. The decrease in heart PDHa in the obese animals was reversed by a single dose of 2-tetradecylglycidic acid, but this inhibitor of mitochondrial fatty acid oxidation did not affect liver PDHa in these animals. These early and diverse changes in PDHa argue for a multifactorial aetiology in the development of the whole-body insulin resistance seen in older gold-thioglucose-treated obese animals.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Assimacopoulos-Jeannet F., Jeanrenaud B. The hormonal and metabolic basis of experimental obesity. Clin Endocrinol Metab. 1976 Jul;5(2):337–365. doi: 10.1016/s0300-595x(76)80025-4. [DOI] [PubMed] [Google Scholar]
- Bray G. A., York D. A. Hypothalamic and genetic obesity in experimental animals: an autonomic and endocrine hypothesis. Physiol Rev. 1979 Jul;59(3):719–809. doi: 10.1152/physrev.1979.59.3.719. [DOI] [PubMed] [Google Scholar]
- Caterson I. D., Fuller S. J., Randle P. J. Effect of the fatty acid oxidation inhibitor 2-tetradecylglycidic acid on pyruvate dehydrogenase complex activity in starved and alloxan-diabetic rats. Biochem J. 1982 Oct 15;208(1):53–60. doi: 10.1042/bj2080053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caterson I. D., Taylor K. W. Islet cell function in gold thioglucose-induced obesity in mice. Diabetologia. 1982 Aug;23(2):119–123. doi: 10.1007/BF01271172. [DOI] [PubMed] [Google Scholar]
- Caterson I. D., Williams P. F., Kerbey A. L., Astbury L. D., Plehwe W. E., Turtle J. R. The effect of body weight and the fatty acid-oxidation inhibitor 2-tetradecylglycidic acid on pyruvate dehydrogenase complex activity in mouse heart. Biochem J. 1984 Dec 15;224(3):787–791. doi: 10.1042/bj2240787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crettaz M., Prentki M., Zaninetti D., Jeanrenaud B. Insulin resistance in soleus muscle from obese Zucker rats. Involvement of several defective sites. Biochem J. 1980 Feb 15;186(2):525–534. doi: 10.1042/bj1860525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golay A., Swislocki A. L., Chen Y. D., Jaspan J. B., Reaven G. M. Effect of obesity on ambient plasma glucose, free fatty acid, insulin, growth hormone, and glucagon concentrations. J Clin Endocrinol Metab. 1986 Aug;63(2):481–484. doi: 10.1210/jcem-63-2-481. [DOI] [PubMed] [Google Scholar]
- Hutson N. J., Randle P. J. Enhanced activity of pyruvate dehydrogenase kinase in rat heart mitochondria in alloxan-diabetes or starvation. FEBS Lett. 1978 Aug 1;92(1):73–76. doi: 10.1016/0014-5793(78)80724-8. [DOI] [PubMed] [Google Scholar]
- KATSUKI S., HIRATA Y., HORINO M., ITO M., ISHIMOTO M., MAKINO N., HOSOSAKO A. Obesity and hyperglycemia induced in mice by goldthioglucose. Diabetes. 1962 May-Jun;11:209–215. [PubMed] [Google Scholar]
- Kerbey A. L., Caterson I. D., Williams P. F., Turtle J. R. Proportion of active dephosphorylated pyruvate dehydrogenase complex in heart and isolated heart mitochondria is decreased in obese hyperinsulinaemic mice. Biochem J. 1984 Jan 1;217(1):117–121. doi: 10.1042/bj2170117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerbey A. L., Randle P. J., Cooper R. H., Whitehouse S., Pask H. T., Denton R. M. Regulation of pyruvate dehydrogenase in rat heart. Mechanism of regulation of proportions of dephosphorylated and phosphorylated enzyme by oxidation of fatty acids and ketone bodies and of effects of diabetes: role of coenzyme A, acetyl-coenzyme A and reduced and oxidized nicotinamide-adenine dinucleotide. Biochem J. 1976 Feb 15;154(2):327–348. doi: 10.1042/bj1540327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Marchand Y., Freychet P., Jeanrenaud B. Longitudinal study on the establishment of insulin resistance in hypothalamic obese mice. Endocrinology. 1978 Jan;102(1):74–85. doi: 10.1210/endo-102-1-74. [DOI] [PubMed] [Google Scholar]
- Linn T. C., Pettit F. H., Hucho F., Reed L. J. Alpha-keto acid dehydrogenase complexes. XI. Comparative studies of regulatory properties of the pyruvate dehydrogenase complexes from kidney, heart, and liver mitochondria. Proc Natl Acad Sci U S A. 1969 Sep;64(1):227–234. doi: 10.1073/pnas.64.1.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munson P. J., Rodbard D. Ligand: a versatile computerized approach for characterization of ligand-binding systems. Anal Biochem. 1980 Sep 1;107(1):220–239. doi: 10.1016/0003-2697(80)90515-1. [DOI] [PubMed] [Google Scholar]
- Olefsky J. M. LIlly lecture 1980. Insulin resistance and insulin action. An in vitro and in vivo perspective. Diabetes. 1981 Feb;30(2):148–162. doi: 10.2337/diab.30.2.148. [DOI] [PubMed] [Google Scholar]
- Palmer W. K., Kane T. A. Hormone-stimulated lipolysis in cardiac myocytes. Biochem J. 1983 Oct 15;216(1):241–243. doi: 10.1042/bj2160241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitehouse S., Cooper R. H., Randle P. J. Mechanism of activation of pyruvate dehydrogenase by dichloroacetate and other halogenated carboxylic acids. Biochem J. 1974 Sep;141(3):761–774. doi: 10.1042/bj1410761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams P. F., Turtle J. R. Purification of the insulin receptor from human placental membranes. Biochim Biophys Acta. 1979 Aug 28;579(2):367–374. doi: 10.1016/0005-2795(79)90064-3. [DOI] [PubMed] [Google Scholar]


