Abstract
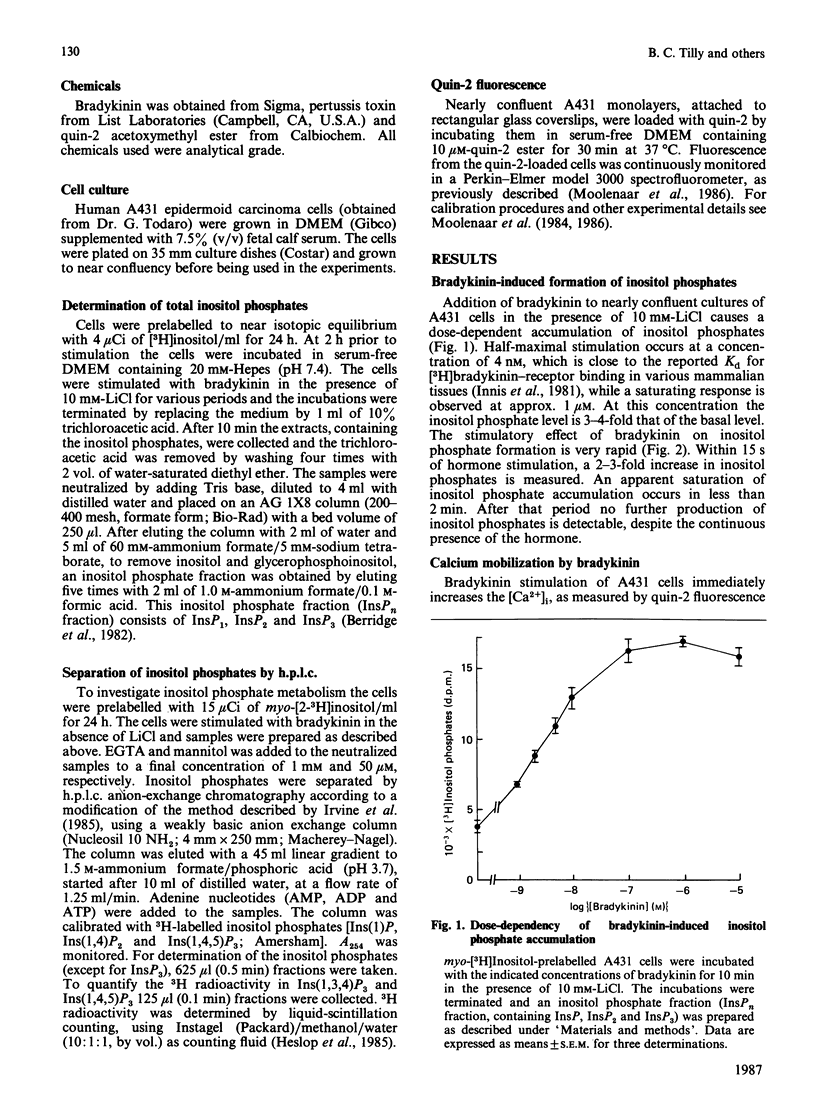
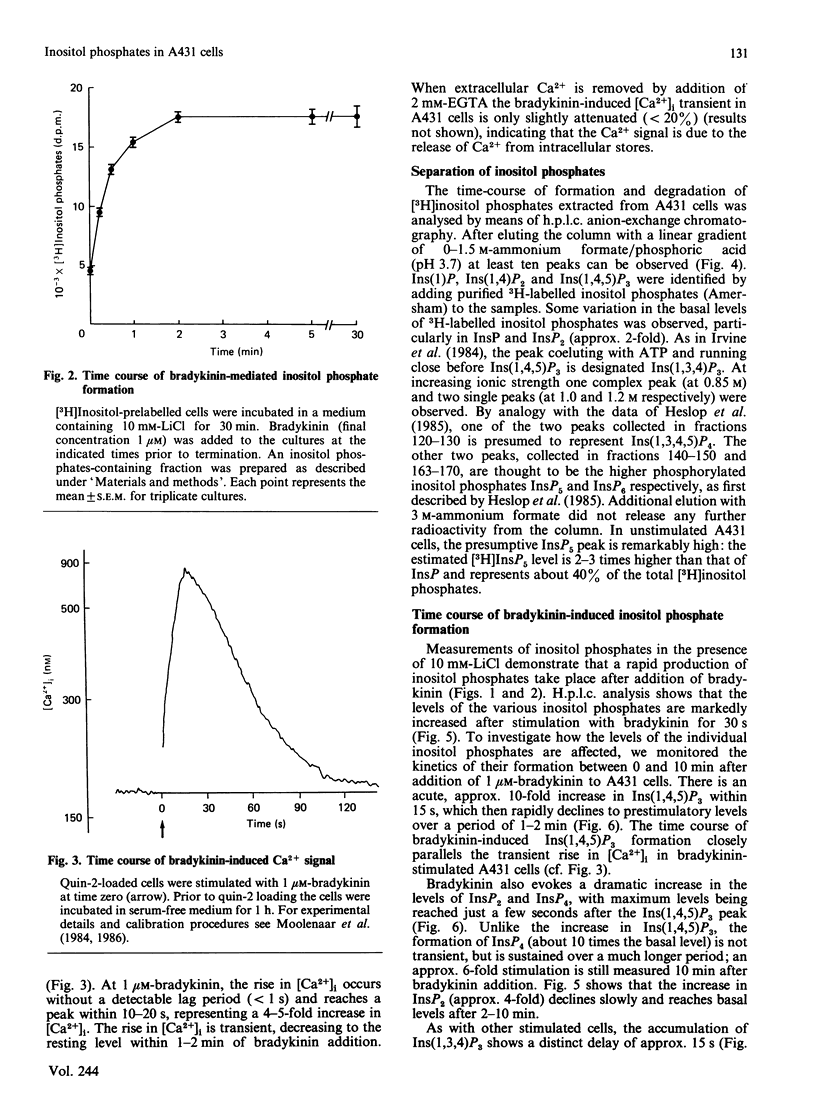
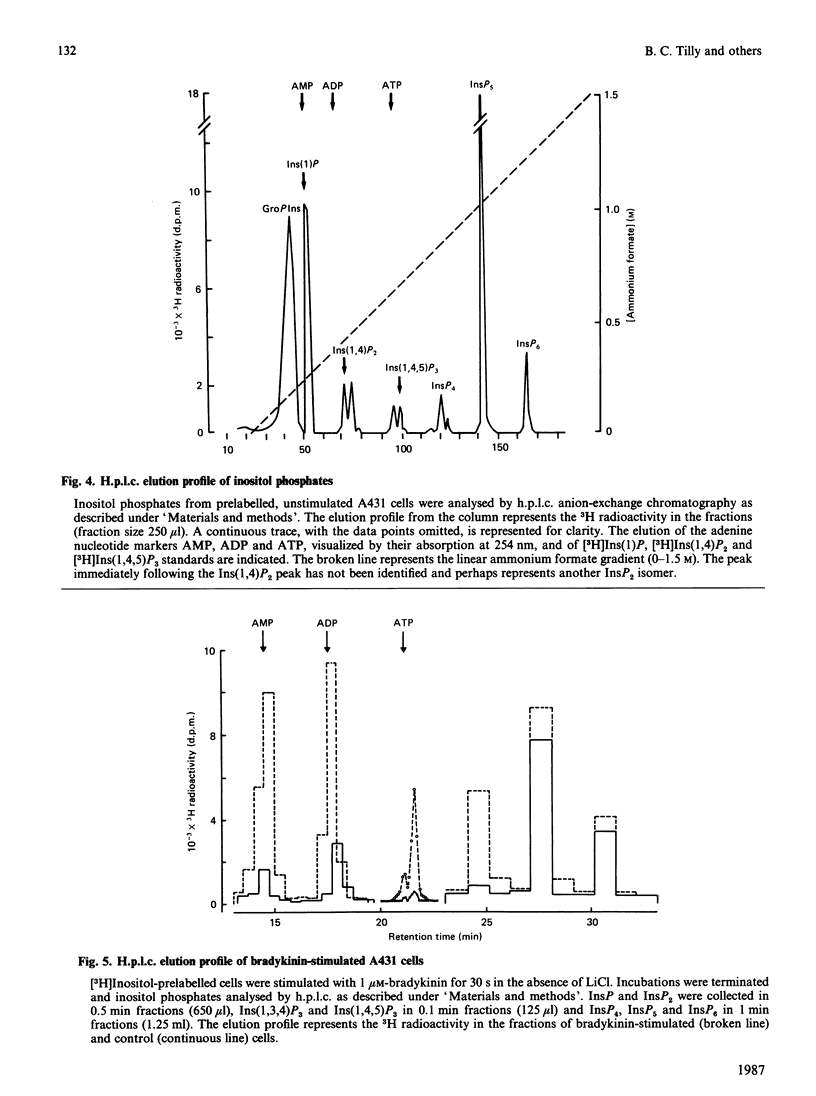
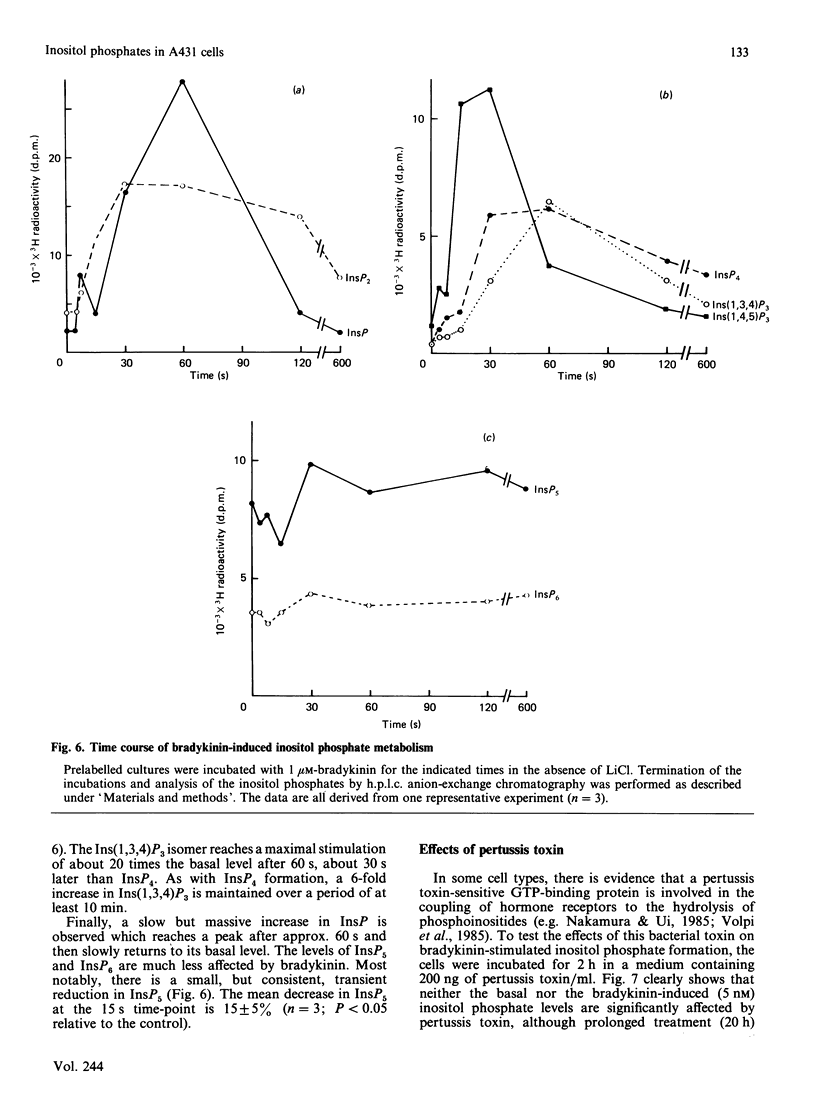
Stimulation of human A431 epidermoid carcinoma cells by bradykinin causes a very rapid release of inositol phosphates and a transient rise in cytoplasmic free Ca2+ concentration ([Ca2+]i). Bradykinin-induced inositol phosphate formation is half-maximal at a concentration of 4 nM and is not affected by pertussis toxin. H.p.l.c. analysis of the various inositol phosphates shows an immediate but transient accumulation of inositol 1,4,5-trisphosphate [Ins(1,4,5)P3], which reaches a peak value of approx. 10 times the basal level within 15 s and slightly precedes the rise in [Ca2+]i, both parameters changing in parallel. After a lag period, bradykinin also induces a massive accumulation of Ins(1,3,4)P3 and inositol 1,3,4,5-tetrakisphosphate [Ins(1,3,4,5)P4]. Our data support the view that part of the newly formed Ins(1,4,5)P3 is converted into Ins(1,3,4)P3 phosphorylation/dephosphorylation with Ins(1,3,4,5)P4 as intermediate. Furthermore, A431 cells were found to contain strikingly high basal levels of two other inositol phosphates, presumably inositol pentakisphosphate (InsP5) and inositol hexakisphosphate (InsP6), representing more than 50% of the total 3H radioactivity incorporated into inositol phosphates. The presumptive InsP5 and InsP6 are only slightly affected by bradykinin. Although Ins(1,3,4)P3 and InsP4 could function as second messengers, our results suggest that, unlike Ins(1,4,5)P3, neither Ins(1,3,4)P3 nor InsP4 are involved in Ca2+ mobilization.
Full text
PDF
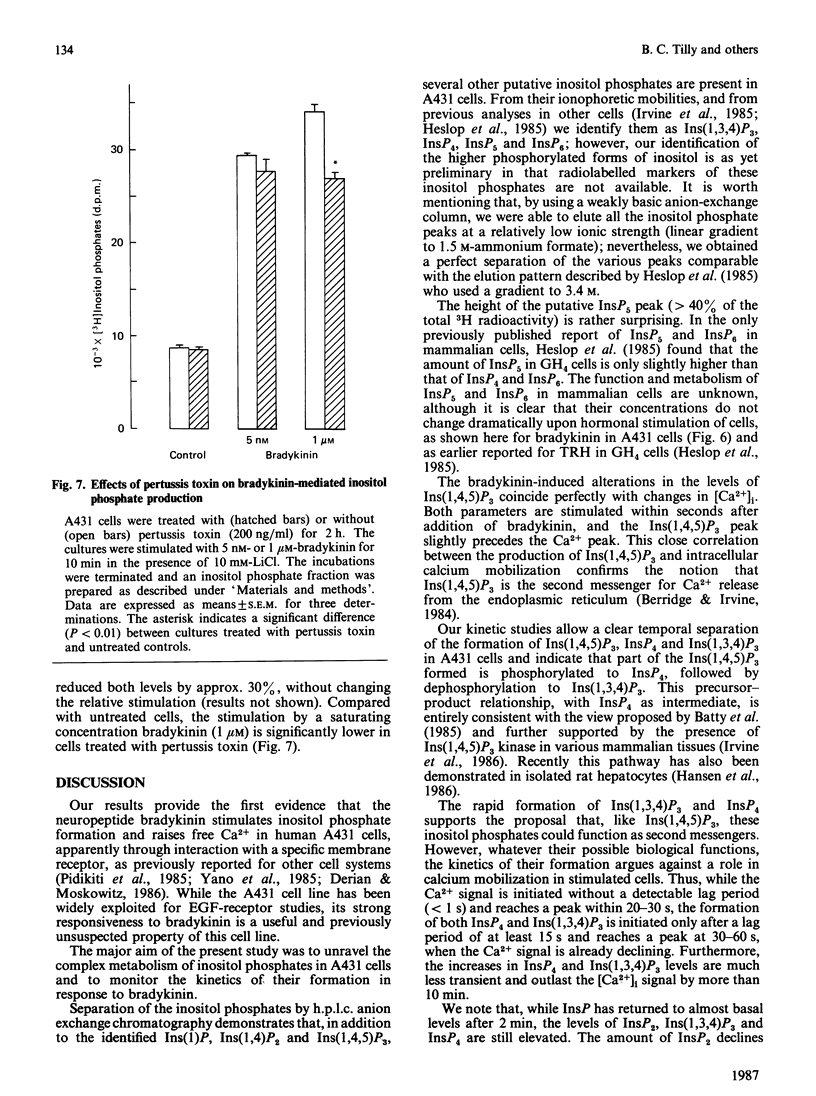

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Batty I. R., Nahorski S. R., Irvine R. F. Rapid formation of inositol 1,3,4,5-tetrakisphosphate following muscarinic receptor stimulation of rat cerebral cortical slices. Biochem J. 1985 Nov 15;232(1):211–215. doi: 10.1042/bj2320211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge M. J., Downes C. P., Hanley M. R. Lithium amplifies agonist-dependent phosphatidylinositol responses in brain and salivary glands. Biochem J. 1982 Sep 15;206(3):587–595. doi: 10.1042/bj2060587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge M. J. Inositol trisphosphate and diacylglycerol as second messengers. Biochem J. 1984 Jun 1;220(2):345–360. doi: 10.1042/bj2200345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge M. J., Irvine R. F. Inositol trisphosphate, a novel second messenger in cellular signal transduction. Nature. 1984 Nov 22;312(5992):315–321. doi: 10.1038/312315a0. [DOI] [PubMed] [Google Scholar]
- Brock T. A., Rittenhouse S. E., Powers C. W., Ekstein L. S., Gimbrone M. A., Jr, Alexander R. W. Phorbol ester and 1-oleoyl-2-acetylglycerol inhibit angiotensin activation of phospholipase C in cultured vascular smooth muscle cells. J Biol Chem. 1985 Nov 15;260(26):14158–14162. [PubMed] [Google Scholar]
- Burgess G. M., McKinney J. S., Irvine R. F., Putney J. W., Jr Inositol 1,4,5-trisphosphate and inositol 1,3,4-trisphosphate formation in Ca2+-mobilizing-hormone-activated cells. Biochem J. 1985 Nov 15;232(1):237–243. doi: 10.1042/bj2320237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derian C. K., Moskowitz M. A. Polyphosphoinositide hydrolysis in endothelial cells and carotid artery segments. Bradykinin-2 receptor stimulation is calcium-independent. J Biol Chem. 1986 Mar 15;261(8):3831–3837. [PubMed] [Google Scholar]
- Hansen C. A., Mah S., Williamson J. R. Formation and metabolism of inositol 1,3,4,5-tetrakisphosphate in liver. J Biol Chem. 1986 Jun 25;261(18):8100–8103. [PubMed] [Google Scholar]
- Heslop J. P., Irvine R. F., Tashjian A. H., Jr, Berridge M. J. Inositol tetrakis- and pentakisphosphates in GH4 cells. J Exp Biol. 1985 Nov;119:395–401. doi: 10.1242/jeb.119.1.395. [DOI] [PubMed] [Google Scholar]
- Hori C., Oka T. Induction by lithium ion of multiplication of mouse mammary epithelium in culture. Proc Natl Acad Sci U S A. 1979 Jun;76(6):2823–2827. doi: 10.1073/pnas.76.6.2823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Innis R. B., Manning D. C., Stewart J. M., Snyder S. H. [3H]Bradykinin receptor binding in mammalian tissue membranes. Proc Natl Acad Sci U S A. 1981 Apr;78(4):2630–2634. doi: 10.1073/pnas.78.4.2630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irvine R. F., Anggård E. E., Letcher A. J., Downes C. P. Metabolism of inositol 1,4,5-trisphosphate and inositol 1,3,4-trisphosphate in rat parotid glands. Biochem J. 1985 Jul 15;229(2):505–511. doi: 10.1042/bj2290505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irvine R. F., Letcher A. J., Heslop J. P., Berridge M. J. The inositol tris/tetrakisphosphate pathway--demonstration of Ins(1,4,5)P3 3-kinase activity in animal tissues. Nature. 1986 Apr 17;320(6063):631–634. doi: 10.1038/320631a0. [DOI] [PubMed] [Google Scholar]
- Irvine R. F., Letcher A. J., Lander D. J., Downes C. P. Inositol trisphosphates in carbachol-stimulated rat parotid glands. Biochem J. 1984 Oct 1;223(1):237–243. doi: 10.1042/bj2230237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leeb-Lundberg L. M., Cotecchia S., Lomasney J. W., DeBernardis J. F., Lefkowitz R. J., Caron M. G. Phorbol esters promote alpha 1-adrenergic receptor phosphorylation and receptor uncoupling from inositol phospholipid metabolism. Proc Natl Acad Sci U S A. 1985 Sep;82(17):5651–5655. doi: 10.1073/pnas.82.17.5651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michell R. H., Kirk C. J., Jones L. M., Downes C. P., Creba J. A. The stimulation of inositol lipid metabolism that accompanies calcium mobilization in stimulated cells: defined characteristics and unanswered questions. Philos Trans R Soc Lond B Biol Sci. 1981 Dec 18;296(1080):123–138. doi: 10.1098/rstb.1981.0177. [DOI] [PubMed] [Google Scholar]
- Moolenaar W. H., Aerts R. J., Tertoolen L. G., de Laat S. W. The epidermal growth factor-induced calcium signal in A431 cells. J Biol Chem. 1986 Jan 5;261(1):279–284. [PubMed] [Google Scholar]
- Moolenaar W. H., Tertoolen L. G., de Laat S. W. Growth factors immediately raise cytoplasmic free Ca2+ in human fibroblasts. J Biol Chem. 1984 Jul 10;259(13):8066–8069. [PubMed] [Google Scholar]
- Nakamura T., Ui M. Simultaneous inhibitions of inositol phospholipid breakdown, arachidonic acid release, and histamine secretion in mast cells by islet-activating protein, pertussis toxin. A possible involvement of the toxin-specific substrate in the Ca2+-mobilizing receptor-mediated biosignaling system. J Biol Chem. 1985 Mar 25;260(6):3584–3593. [PubMed] [Google Scholar]
- Nishizuka Y. The role of protein kinase C in cell surface signal transduction and tumour promotion. Nature. 1984 Apr 19;308(5961):693–698. doi: 10.1038/308693a0. [DOI] [PubMed] [Google Scholar]
- Orellana S. A., Solski P. A., Brown J. H. Phorbol ester inhibits phosphoinositide hydrolysis and calcium mobilization in cultured astrocytoma cells. J Biol Chem. 1985 May 10;260(9):5236–5239. [PubMed] [Google Scholar]
- Owen N. E., Villereal M. L. Lys-bradykinin stimulates Na+ influx and DNA synthesis in cultured human fibroblasts. Cell. 1983 Mar;32(3):979–985. doi: 10.1016/0092-8674(83)90082-x. [DOI] [PubMed] [Google Scholar]
- Pidikiti N., Gamero D., Gamero J., Hassid A. Bradykinin-evoked modulation of cytosolic Ca2+ concentrations in cultured renal epithelial (MDCK) cells. Biochem Biophys Res Commun. 1985 Jul 31;130(2):807–813. doi: 10.1016/0006-291x(85)90488-7. [DOI] [PubMed] [Google Scholar]
- Ptashne K., Stockdale F. E., Conlon S. Initiation of DNA synthesis in mammary epithelium and mammary tumors by lithium ions. J Cell Physiol. 1980 Apr;103(1):41–46. doi: 10.1002/jcp.1041030107. [DOI] [PubMed] [Google Scholar]
- Rybak S. M., Stockdale F. E. Growth effects of lithium chloride in BALB/c 3T3 fibroblasts and Madin-Darby canine kidney epithelial cells. Exp Cell Res. 1981 Dec;136(2):263–270. doi: 10.1016/0014-4827(81)90004-5. [DOI] [PubMed] [Google Scholar]
- Sano K., Takai Y., Yamanishi J., Nishizuka Y. A role of calcium-activated phospholipid-dependent protein kinase in human platelet activation. Comparison of thrombin and collagen actions. J Biol Chem. 1983 Feb 10;258(3):2010–2013. [PubMed] [Google Scholar]
- Sawyer S. T., Cohen S. Enhancement of calcium uptake and phosphatidylinositol turnover by epidermal growth factor in A-431 cells. Biochemistry. 1981 Oct 13;20(21):6280–6286. doi: 10.1021/bi00524a057. [DOI] [PubMed] [Google Scholar]
- Straus D. S., Pang K. J. Effects of bradykinin on DNA synthesis in resting NIL8 hamster cells and human fibroblasts. Exp Cell Res. 1984 Mar;151(1):87–95. doi: 10.1016/0014-4827(84)90358-6. [DOI] [PubMed] [Google Scholar]
- Takai Y., Kaibuchi K., Sano K., Nishizuka Y. Counteraction of calcium-activated, phospholipid-dependent protein kinase activation by adenosine 3',5'-monophosphate and guanosine 3',5'-monophosphate in platelets. J Biochem. 1982 Jan;91(1):403–406. doi: 10.1093/oxfordjournals.jbchem.a133700. [DOI] [PubMed] [Google Scholar]
- Volpi M., Naccache P. H., Molski T. F., Shefcyk J., Huang C. K., Marsh M. L., Munoz J., Becker E. L., Sha'afi R. I. Pertussis toxin inhibits fMet-Leu-Phe- but not phorbol ester-stimulated changes in rabbit neutrophils: role of G proteins in excitation response coupling. Proc Natl Acad Sci U S A. 1985 May;82(9):2708–2712. doi: 10.1073/pnas.82.9.2708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yano K., Higashida H., Hattori H., Nozawa Y. Bradykinin-induced transient accumulation of inositol trisphosphate in neuron-like cell line NG108-15 cells. FEBS Lett. 1985 Feb 25;181(2):403–406. doi: 10.1016/0014-5793(85)80301-x. [DOI] [PubMed] [Google Scholar]


