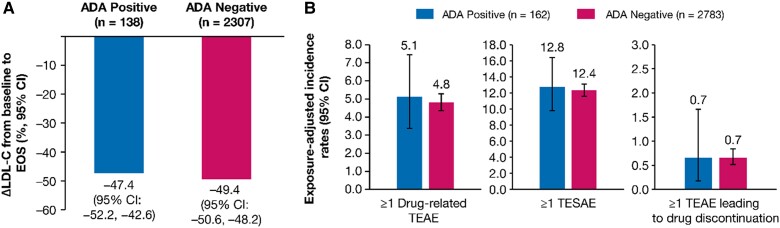
Figure 6.
Efficacy and safety by ADA Status. (A) Mean percentage change in LDL-C from baseline to EOS, and (B) Exposure-adjusted incidence rate (per 100 patient-years) for TEAE and TESAE. The ADA response was determined from the pooled analysis of ORION-3, ORION-9, ORION-10, ORION-11, and ORION-8 ADA data. ORION-1 inclisiran arm patients were excluded from the analysis because the laboratory protocol used in the ORION-1 ADA assessment was different from other studies. The exposure-adjusted incidence rates of TEAEs were calculated since the first inclisiran injection across the parent and extension studies. Δ, mean percentage change; ADA, antidrug antibody; EOS, end of study; LDL-C, low-density lipoprotein cholesterol; TEAE, treatment-emergent adverse event; TESAE, treatment-emergent serious adverse event.