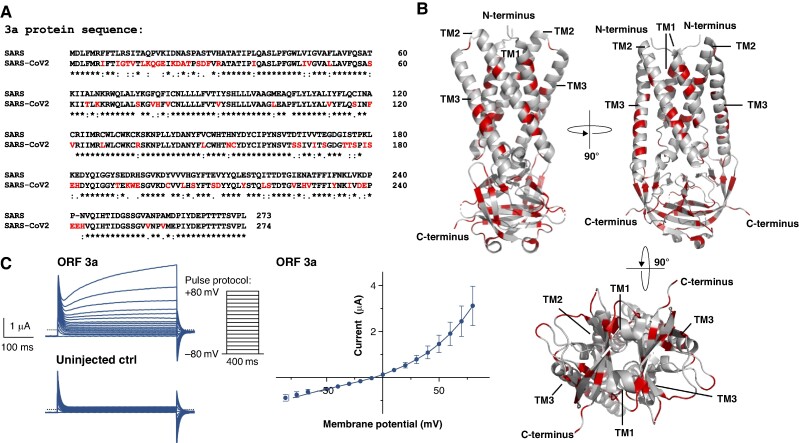
Figure 2.
The SARS-CoV-2 ORF 3a protein gives rise to outward potassium currents upon heterologous expression in X. laevis oocytes. (A) Protein sequence alignment of the ORF 3a protein sequences derived from SARS-CoV-2 and the classic SARS-CoV-1 variant (SARS2018). Differences in the amino acid sequence are highlighted in red *, conserved amino acid residue; :, conservative amino acid residue exchange; ., semiconservative amino acid residue exchange. (B) 3D visualization of the SARS-CoV-2 ORF 3a dimer based on the cryo-EM structure recently revealed by Kern et al.22 (PDB-ID: 6XDC); again the differences to the SARS-CoV-1 variant are highlighted in red. TM1–3, transmembrane domain 1–3. (C) Representative family of current trances recorded from X. laevis oocytes, heterologously expressing SARS-CoV-2 ORF 3a proteins by application of the pulse protocol depicted and respective uninjected control (ctrl) oocytes. Current–voltage relationship of SARS-CoV-2 ORF 3a protein-mediated current. Current amplitudes of ORF 3a-expressing X. laevis oocytes were quantified at the end of each 400 ms voltage pulse. Data are expressed as mean ± standard error of the mean from n = 5 cells. Dotted line represents zero current level, and scale bar is depicted as inset.