Abstract
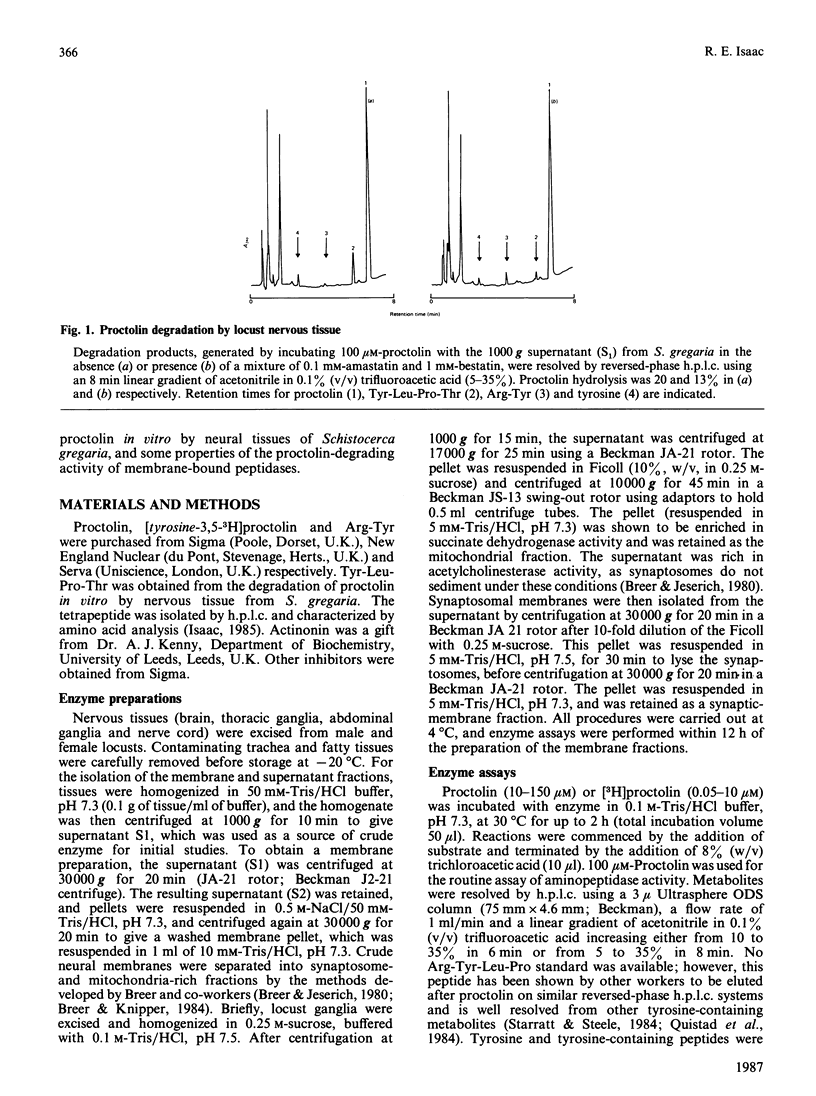
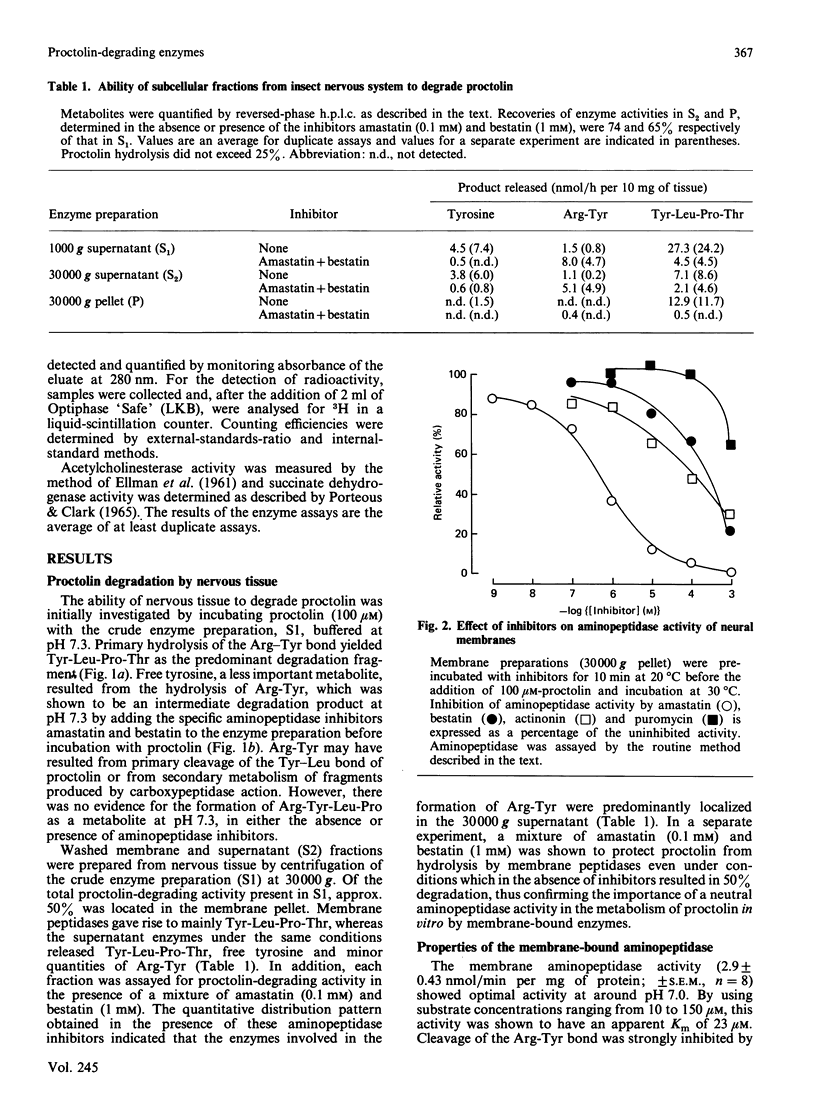
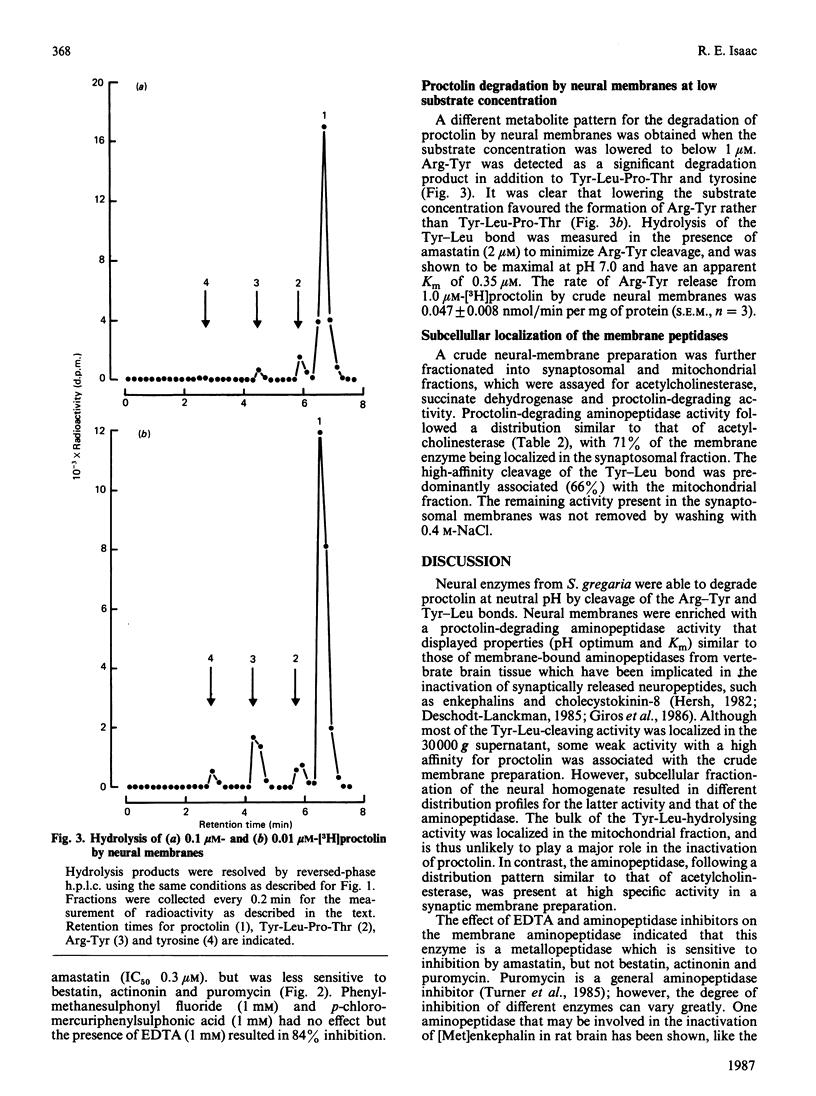
The hydrolysis of the insect neuropeptide proctolin (Arg-Tyr-Leu-Pro-Thr) by enzyme preparations from the nervous tissue of the desert locust (Schistocerca gregaria) was investigated. Neural homogenate degraded proctolin (100 microM) at neutral pH by cleavage of the Arg-Tyr and Tyr-Leu bonds to yield Tyr-Leu-Pro-Thr, Arg-Tyr and free tyrosine. Arg-Tyr was detected as a major metabolite when the aminopeptidase inhibitors amastatin and bestatin were present to prevent Arg-Tyr breakdown. Around 50% of the proctolin-degrading activity was isolated in a 30,000 g membrane fraction and was shown to be almost entirely due to aminopeptidase activity. The aminopeptidase had an apparent Km of 23 microM, a pH optimum of 7.0 and was inhibited by 1 mM-EDTA and amastatin [IC50 = 0.3 microM], but was relatively insensitive to bestatin, actinonin and puromycin. Phenylmethanesulphonyl fluoride (1 mM) and p-chloromercuriphenylsulphonic acid (1 mM) had no effect on this enzyme activity. Although the bulk of the Tyr-Leu hydrolytic activity was located in the 30,000 g supernatant, some weak activity was detected in a washed membrane preparation. This peptidase displayed a high affinity for proctolin (Km = 0.35 microM) and optimal activity at around pH 7.0. Synaptosome- and mitochondria-rich fractions were prepared from crude neural membranes. The aminopeptidase activity was concentrated in the synaptic-membrane preparation, whereas activity giving rise to Arg-Tyr was predominantly localized in the mitochondrial fraction. The subcellular localization of the membrane aminopeptidase is consistent with a possible physiological role for this enzyme in the inactivation of synaptically released proctolin.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bishop C. A., O'Shea M., Miller R. J. Neuropeptide proctolin (H-Arg-Tyr-Leu-Pro-Thr-OH): immunological detection and neuronal localization in insect central nervous system. Proc Natl Acad Sci U S A. 1981 Sep;78(9):5899–5902. doi: 10.1073/pnas.78.9.5899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown B. E. Proctolin: a peptide transmitter candidate in insects. Life Sci. 1975 Oct 15;17(8):1241–1252. doi: 10.1016/0024-3205(75)90133-2. [DOI] [PubMed] [Google Scholar]
- Deschodt-Lanckman M. Enzymatic degradation of cholecystokinin in the central nervous system. Ann N Y Acad Sci. 1985;448:87–98. doi: 10.1111/j.1749-6632.1985.tb29909.x. [DOI] [PubMed] [Google Scholar]
- ELLMAN G. L., COURTNEY K. D., ANDRES V., Jr, FEATHER-STONE R. M. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharmacol. 1961 Jul;7:88–95. doi: 10.1016/0006-2952(61)90145-9. [DOI] [PubMed] [Google Scholar]
- Eckert M., Agricola H., Penzlin H. Immunocytochemical identification of proctolinlike immunoreactivity in the terminal ganglion and hindgut of the cockroach Periplaneta americana (L.). Cell Tissue Res. 1981;217(3):633–645. doi: 10.1007/BF00219370. [DOI] [PubMed] [Google Scholar]
- Fulcher I. S., Matsas R., Turner A. J., Kenny A. J. Kidney neutral endopeptidase and the hydrolysis of enkephalin by synaptic membranes show similar sensitivity to inhibitors. Biochem J. 1982 May 1;203(2):519–522. doi: 10.1042/bj2030519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giros B., Gros C., Solhonne B., Schwartz J. C. Characterization of aminopeptidases responsible for inactivating endogenous (Met5)enkephalin in brain slices using peptidase inhibitors and anti-aminopeptidase M antibodies. Mol Pharmacol. 1986 Mar;29(3):281–287. [PubMed] [Google Scholar]
- Hersh L. B. Characterization of membrane-bound aminopeptidases from rat brain: identification of the enkephalin-degrading aminopeptidase. J Neurochem. 1985 May;44(5):1427–1435. doi: 10.1111/j.1471-4159.1985.tb08779.x. [DOI] [PubMed] [Google Scholar]
- Hersh L. B. Degradation of enkephalins: the search for an enkephalinase. Mol Cell Biochem. 1982 Aug 20;47(1):35–43. doi: 10.1007/BF00241564. [DOI] [PubMed] [Google Scholar]
- Hersh L. B. Solubilization and characterization of two rat brain membrane-bound aminopeptidases active on Met-enkephalin. Biochemistry. 1981 Apr 14;20(8):2345–2350. doi: 10.1021/bi00511a042. [DOI] [PubMed] [Google Scholar]
- Hudgin R. L., Charleson S. E., Zimmerman M., Mumford R., Wood P. L. Enkephalinase: selective peptide inhibitors. Life Sci. 1981 Dec 21;29(25):2593–2601. doi: 10.1016/0024-3205(81)90632-9. [DOI] [PubMed] [Google Scholar]
- Krieger D. T. Brain peptides: what, where, and why? Science. 1983 Dec 2;222(4627):975–985. doi: 10.1126/science.6139875. [DOI] [PubMed] [Google Scholar]
- Matsas R., Fulcher I. S., Kenny A. J., Turner A. J. Substance P and [Leu]enkephalin are hydrolyzed by an enzyme in pig caudate synaptic membranes that is identical with the endopeptidase of kidney microvilli. Proc Natl Acad Sci U S A. 1983 May;80(10):3111–3115. doi: 10.1073/pnas.80.10.3111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsas R., Kenny A. J., Turner A. J. The metabolism of neuropeptides. The hydrolysis of peptides, including enkephalins, tachykinins and their analogues, by endopeptidase-24.11. Biochem J. 1984 Oct 15;223(2):433–440. doi: 10.1042/bj2230433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PORTEOUS J. W., CLARK B. THE ISOLATION AND CHARACTERIZATION OF SUBCELLULAR COMPONENTS OF THE EPITHELIAL CELLS OF RABBIT SMALL INTESTINE. Biochem J. 1965 Jul;96:159–171. doi: 10.1042/bj0960159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quistad G. B., Adams M. E., Scarborough R. M., Carney R. L., Schooley D. A. Metabolism of proctolin, a pentapeptide neurotransmitter in insects. Life Sci. 1984 Feb 6;34(6):569–576. doi: 10.1016/0024-3205(84)90490-9. [DOI] [PubMed] [Google Scholar]
- Schwartz J. C., Malfroy B., De La Baume S. Biological inactivation of enkephalins and the role of enkephalin-dipeptidyl-carboxypeptidase ("enkephalinase") as neuropeptidase. Life Sci. 1981 Oct 26;29(17):1715–1740. doi: 10.1016/0024-3205(81)90182-x. [DOI] [PubMed] [Google Scholar]
- Starratt A. N., Brown B. E. Analogs of the insect myotropic peptide proctolin: synthesis and structure-activity studies. Biochem Biophys Res Commun. 1979 Oct 29;90(4):1125–1130. doi: 10.1016/0006-291x(79)91152-5. [DOI] [PubMed] [Google Scholar]
- Starratt A. N., Brown B. E. Structure of the pentapeptide proctolin, a proposed neurotransmitter in insects. Life Sci. 1975 Oct 15;17(8):1253–1256. doi: 10.1016/0024-3205(75)90134-4. [DOI] [PubMed] [Google Scholar]
- Turner A. J., Matsas R., Kenny A. J. Are there neuropeptide-specific peptidases? Biochem Pharmacol. 1985 May 1;34(9):1347–1356. doi: 10.1016/0006-2952(85)90669-0. [DOI] [PubMed] [Google Scholar]
- Umezawa H., Aoyagi T., Tanaka T., Suda H., Okuyama A., Naganawa H., Hamada M., Takeuchi T. Production of actinonin, an inhibitor of aminopeptidase M, by actinomycetes. J Antibiot (Tokyo) 1985 Nov;38(11):1629–1630. doi: 10.7164/antibiotics.38.1629. [DOI] [PubMed] [Google Scholar]


