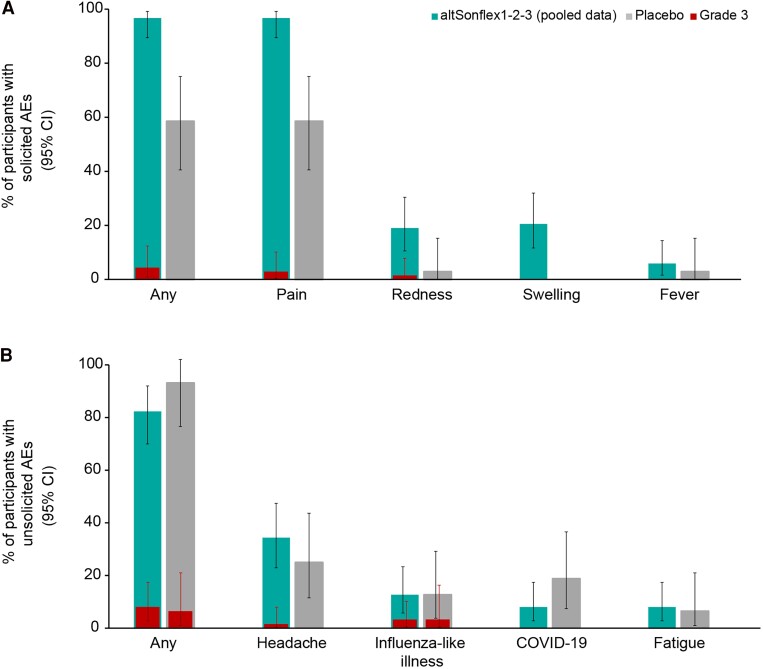
Figure 1.
Percentage of participants with (A) solicited AEs and (B) unsolicited AEs. Abbreviations: AE, adverse event; altSonflex, participants randomized to receive altSonflex1-2-3 vaccine at 3- or 6-month interval (n = 68, pooled data); CI, confidence interval; placebo, participants randomized to receive placebo (n = 34).