Figure 6.
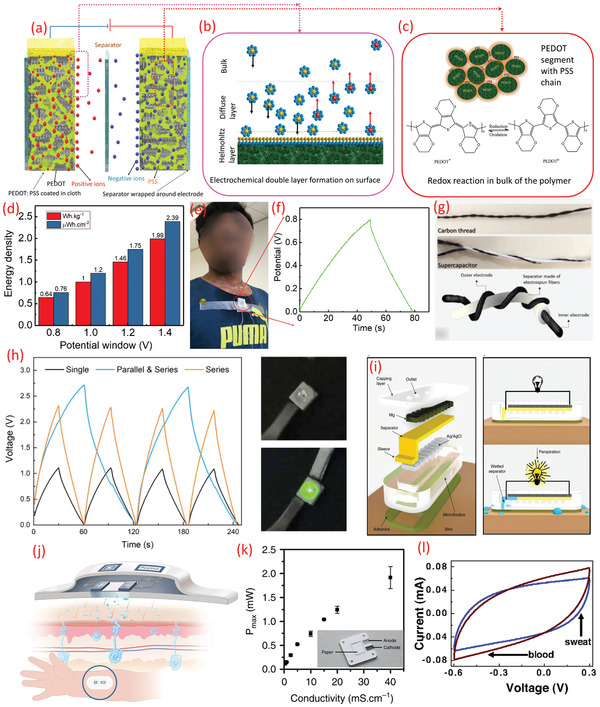
a) Schematic of textile‐based electrodes for SC using sweat as an electrolyte. b) Electrochemical double layer formation on the surface, c) redox reaction on the bulk of the polymer. d) The variation of the energy density of the textile SC (sweat electrolyte) with operating potential window, e) image of the SC on the cloth during exercise. f) The galvanostatic charging–discharging analysis of the textile SC using real sweat. g) Image of twisted configuration wire‐based SC working using sweat equivalent electrolyte. h) Comparison of charge/discharge cycles performances for the combination of SC and its working demonstrated by lighting the LED. i) Exploded view of the sweat‐activated cell with working principle. j) Schematic of the paper battery and its theoretical working. k) The maximum power output density of the paper battery (inset) with the conductivity of the solution. l) CV spectrum of the nanocellulose paper with MWCNT‐based SC in sweat and blood electrolyte. a–f) Adapted under the terms of the CC‐BY Creative Commons Attribution 4.0 International license (https://creativecommons.org/licenses/by/4.0).[ 35 ] Copyright 2020, The Authors, published by Wiley‐VCH. g,h) Reproduced under the terms of the CC‐BY Creative Commons Attribution 4.0 International license (https://creativecommons.org/licenses/by/4.0).[ 78 ] Copyright 2020, The Authors, published by Springer Nature. i) Reproduced with permission.[ 79 ] Copyright 2016, The Authors, published by Springer Nature. j,k) Reproduced under the terms of the CC‐BY Creative Commons Attribution 4.0 International license (https://creativecommons.org/licenses/by/4.0).[ 198 ] Copyright 2019, The Authors, published by Springer Nature. l) Reproduced with permission.[ 199 ] Copyright 2007, National Academy of Sciences.
