Abstract
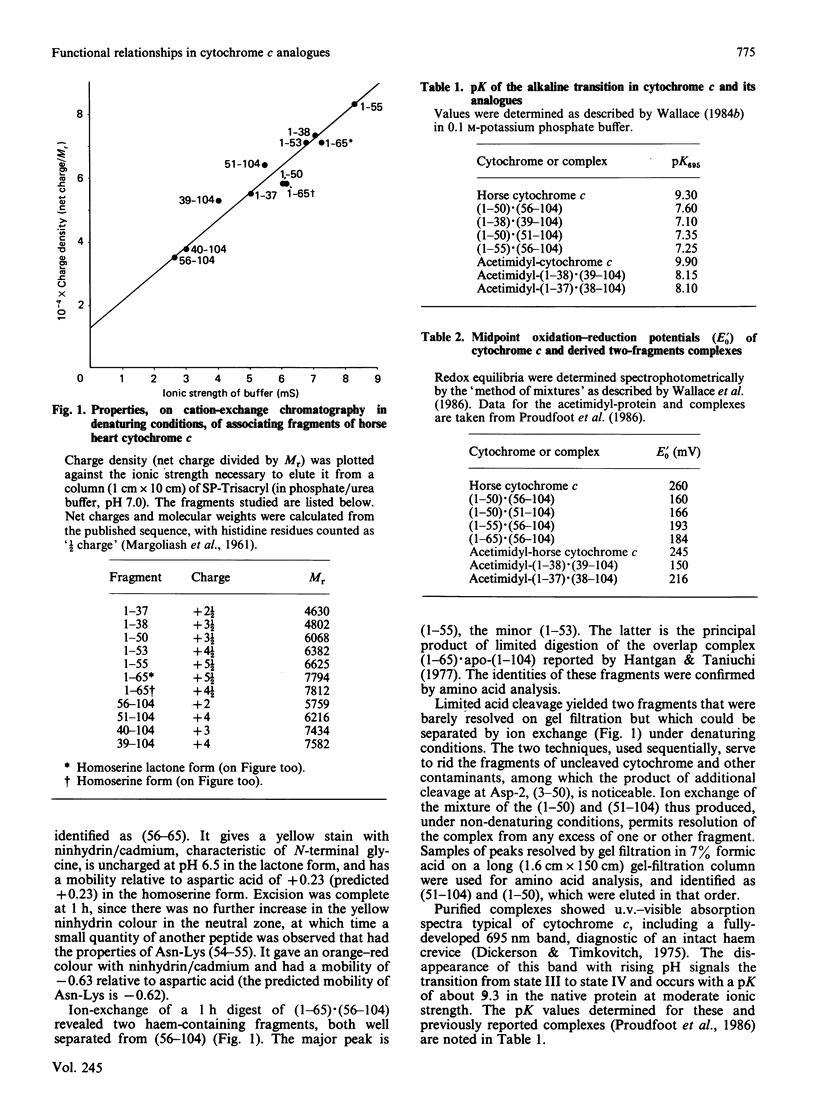
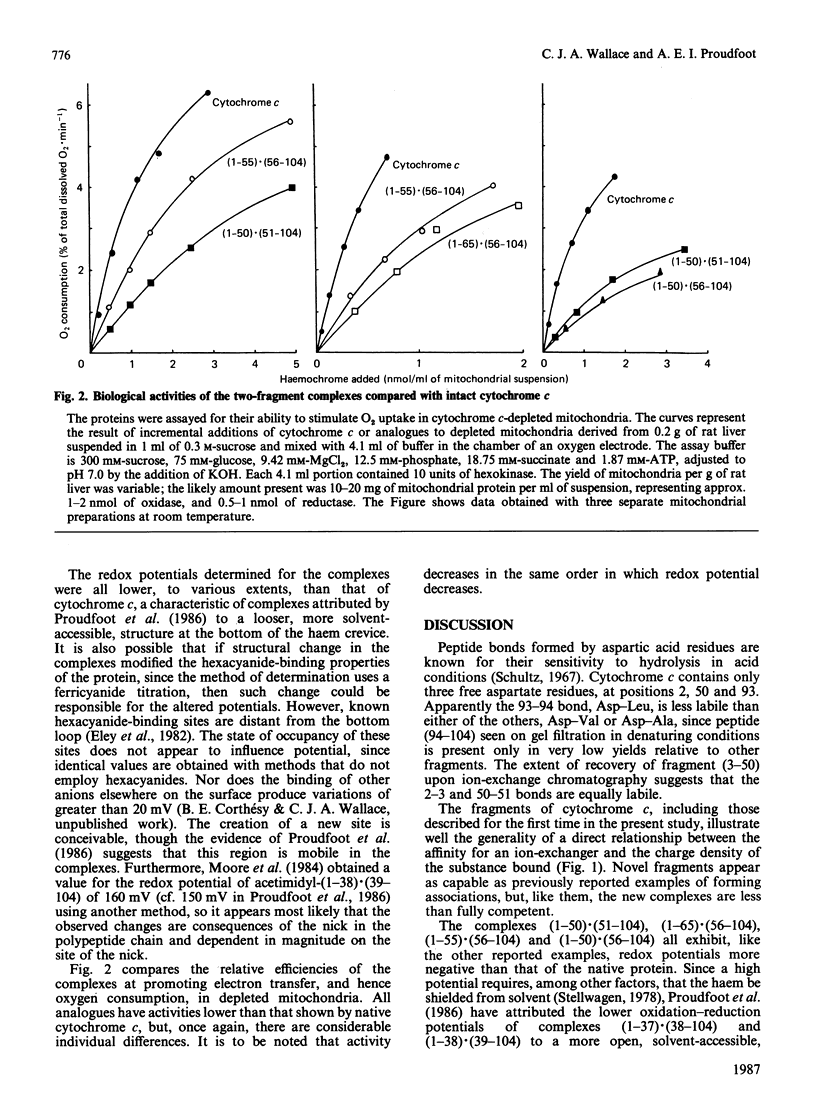
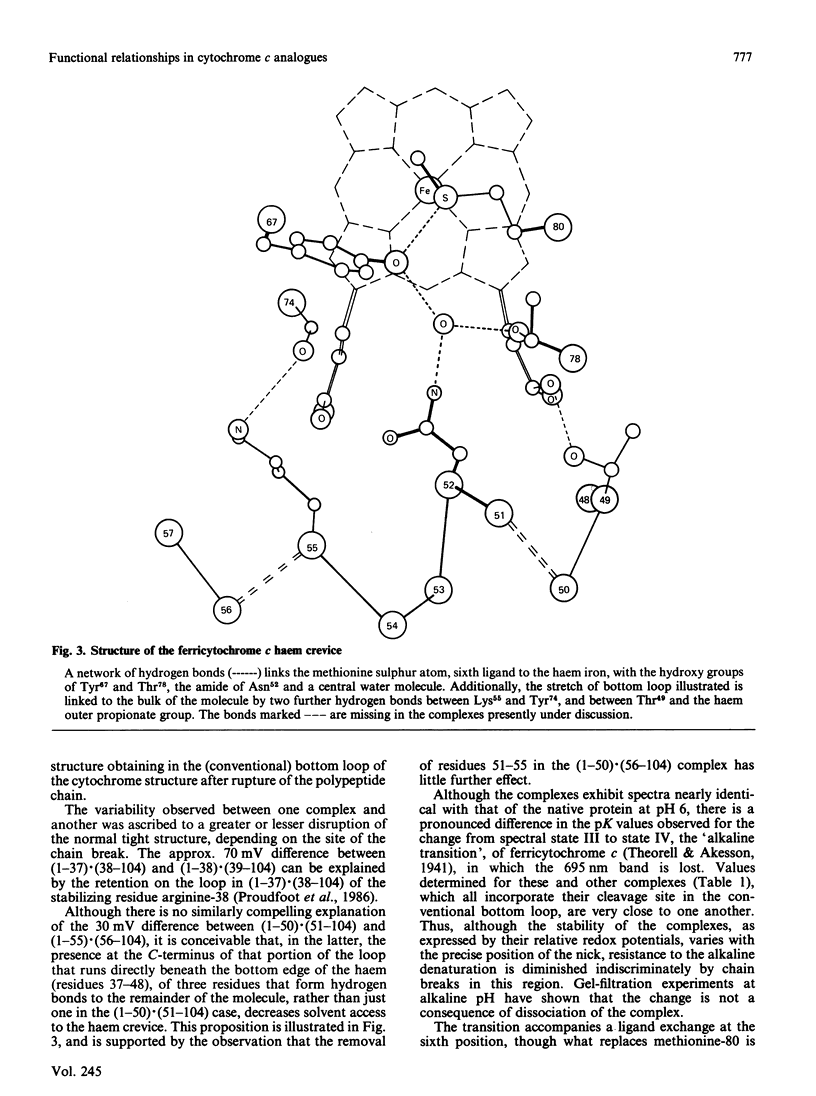
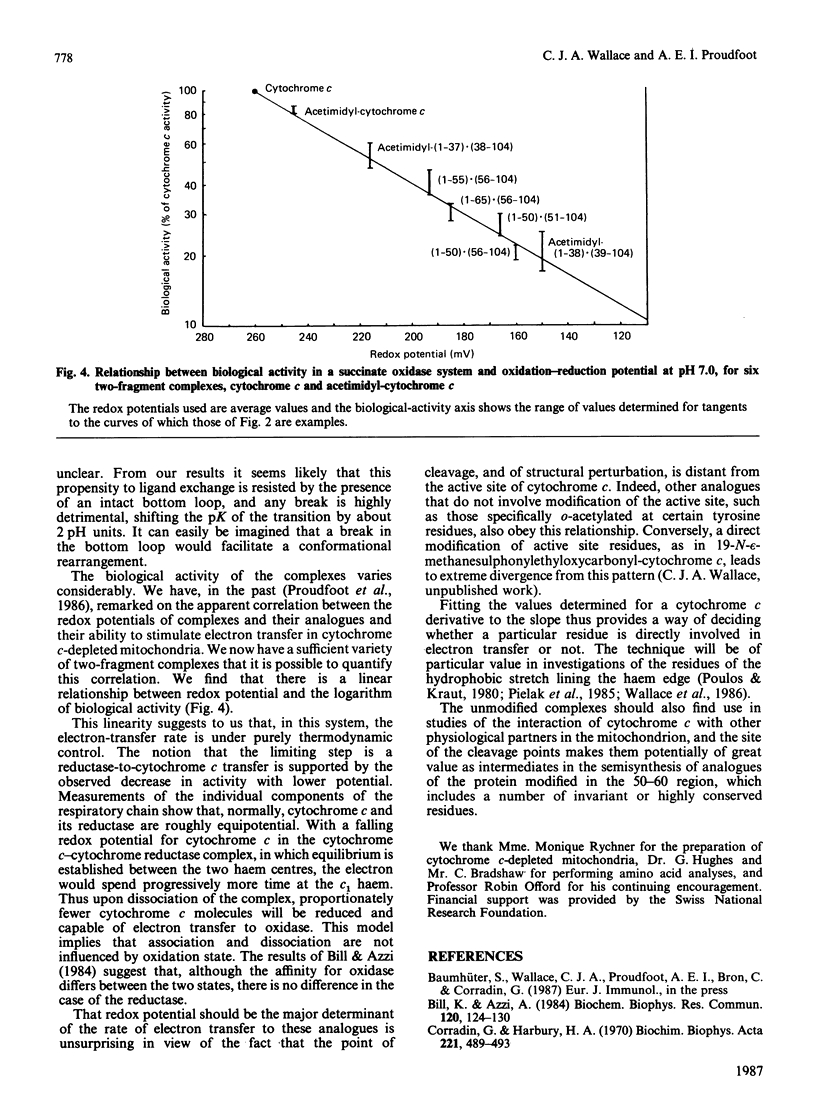
We have confirmed the propensity of fragments of cytochrome c to form complexes that reproduce the structure and, in part, the functionality, of the native protein by preparing four novel complexes. We have used trypsin under three different sets of conditions in sequence to prepare a contiguous two-fragment complex (1-55).(56-104). One of the intermediates is a stable overlapping complex (1-65).(56-104). Conditions for limited acid hydrolysis of peptide bonds in cytochrome c have been developed that optimize the yield of fragments (1-50) and (51-104). These two fragments also form a stable association, as do (1-50) and (56-104). These complexes are potentially useful for the semisynthesis of analogues modified in the region of the cleavage sites, which include a number of highly conserved amino acid residues, and are being used for studies of protein folding, interactions with oxidase, cytochrome c immunogenicity and of artificially induced spontaneous resyntheses between complexing fragments. Like other known two-fragment complexes of cytochrome c, they exhibit normal visible spectra, including the presence of the 695 nm band, indicative of a functional haem crevice. Studies of their biological activities and redox potentials lead to a number of conclusions on structure-function relationships in cytochrome c. Most significantly there is a linear relationship between the logarithm of electron-transfer rates from cytochrome c reductase and redox potential in this series of analogues, indicating that such transfer is thermodynamically controlled. This discovery contributes to our understanding of the interaction of cytochrome and reductase. Since the relationship is obeyed by other types of analogues, except for those that involve modification of the active site of cytochrome c, we have a useful diagnostic for those residues that participate directly in electron transfer.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bill K., Azzi A. Interaction of reduced and oxidized cytochrome c with the mitochondrial cytochrome c oxidase and bc1-complex. Biochem Biophys Res Commun. 1984 Apr 16;120(1):124–130. doi: 10.1016/0006-291x(84)91422-0. [DOI] [PubMed] [Google Scholar]
- Corradin G., Harbury H. A. Cleavage of cytochrome c with cyanogen bromide. Biochim Biophys Acta. 1970 Dec 22;221(3):489–496. doi: 10.1016/0005-2795(70)90219-9. [DOI] [PubMed] [Google Scholar]
- Corradin G., Harbury H. A. Reconstitution of horse heart cytochrome c: interaction of the components obtained upon cleavage of the peptide bond following methionine residue 65. Proc Natl Acad Sci U S A. 1971 Dec;68(12):3036–3039. doi: 10.1073/pnas.68.12.3036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corradin G., Harbury H. A. Reconstitution of horse heart cytochrome c: reformation of the peptide bond linking residues 65 and 66. Biochem Biophys Res Commun. 1974 Dec 23;61(4):1400–1406. doi: 10.1016/s0006-291x(74)80439-0. [DOI] [PubMed] [Google Scholar]
- Eley C. G., Moore G. R., Williams G., Williams R. J. 1H NMR studies of the electron exchange between cytochrome c and iron hexacyanides. Definition of the iron hexacyanide binding sites on cytochrome c. Eur J Biochem. 1982 May 17;124(2):295–303. doi: 10.1111/j.1432-1033.1982.tb06591.x. [DOI] [PubMed] [Google Scholar]
- Hantgan R. R., Taniuchi H. Conformational dynamics in cytochrome c. A fragment exchange study. J Biol Chem. 1978 Aug 10;253(15):5373–5380. [PubMed] [Google Scholar]
- Hantgan R. R., Taniuchi H. Formation of a biologically active, ordered complex from two overlapping fragments of cytochrome c. J Biol Chem. 1977 Feb 25;252(4):1367–1374. [PubMed] [Google Scholar]
- Harris D. E., Offord R. E. A functioning complex between tryptic fragments of cytochrome c. A route to the production of semisynthetic analogues. Biochem J. 1977 Jan 1;161(1):21–25. doi: 10.1042/bj1610021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JACOBS E. E., SANADI D. R. The reversible removal of cytochrome c from mitochondria. J Biol Chem. 1960 Feb;235:531–534. [PubMed] [Google Scholar]
- MARGOLIASH E., SMITH E. L., KREIL G., TUPPY H. Amino-acid sequence of horse heart cytochrome c. Nature. 1961 Dec 23;192:1125–1127. doi: 10.1038/1921125a0. [DOI] [PubMed] [Google Scholar]
- Parr G. R., Hantgan R. R., Taniuchi H. Formation of two alternative complementing structures from cytochrome c heme fragment (residue 1 to 38) and the apoprotein. J Biol Chem. 1978 Aug 10;253(15):5381–5388. [PubMed] [Google Scholar]
- Parr G. R., Taniuchi H. A kinetic study of the formation of ordered complexes of ferric cytochrome c fragments. J Biol Chem. 1979 Jun 10;254(11):4836–4842. [PubMed] [Google Scholar]
- Pielak G. J., Mauk A. G., Smith M. Site-directed mutagenesis of cytochrome c shows that an invariant Phe is not essential for function. Nature. 1985 Jan 10;313(5998):152–154. doi: 10.1038/313152a0. [DOI] [PubMed] [Google Scholar]
- Poulos T. L., Kraut J. A hypothetical model of the cytochrome c peroxidase . cytochrome c electron transfer complex. J Biol Chem. 1980 Nov 10;255(21):10322–10330. [PubMed] [Google Scholar]
- Proudfoot A. E., Offord R. E., Rose K., Schmidt M., Wallace C. J. A case of spurious product formation during attempted resynthesis of proteins by reverse proteolysis. Some batches of 'pure' glycerol contain cross-linking agents. Biochem J. 1984 Jul 15;221(2):325–331. doi: 10.1042/bj2210325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proudfoot A. E., Wallace C. J., Harris D. E., Offord R. E. A new non-covalent complex of semisynthetically modified tryptic fragments of cytochrome c. Biochem J. 1986 Oct 15;239(2):333–337. doi: 10.1042/bj2390333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stellwagen E. Haem exposure as the determinate of oxidation-reduction potential of haem proteins. Nature. 1978 Sep 7;275(5675):73–74. doi: 10.1038/275073a0. [DOI] [PubMed] [Google Scholar]
- Takano T., Dickerson R. E. Conformation change of cytochrome c. II. Ferricytochrome c refinement at 1.8 A and comparison with the ferrocytochrome structure. J Mol Biol. 1981 Nov 25;153(1):95–115. doi: 10.1016/0022-2836(81)90529-5. [DOI] [PubMed] [Google Scholar]
- Wallace C. J., Corradin G., Marchiori F., Borin G. Cytochrome c chimerae from natural and synthetic fragments: significance of the biological properties. Biopolymers. 1986 Nov;25(11):2121–2132. doi: 10.1002/bip.360251107. [DOI] [PubMed] [Google Scholar]
- Wallace C. J., Harris D. E. The preparation of fully N-epsilon-acetimidylated cytochrome c. Biochem J. 1984 Feb 1;217(3):589–594. doi: 10.1042/bj2170589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace C. J. Modulation of the alkaline transition in cytochrome c and cytochrome c-T by full or specific partial acetimidylation. Biochem J. 1984 Feb 1;217(3):601–604. doi: 10.1042/bj2170601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace C. J., Offord R. E. The semisynthesis of fragments corresponding to residues 66-104 of horse heart cytochrome c. Biochem J. 1979 Apr 1;179(1):169–182. doi: 10.1042/bj1790169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace C. J., Rose K. The semisynthesis of analogues of cytochrome c. Modifications of arginine residues 38 and 91. Biochem J. 1983 Dec 1;215(3):651–658. doi: 10.1042/bj2150651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace C. J. The effect of complete or specific partial acetimidylation on the biological properties of cytochrome c and cytochrome c-T. Biochem J. 1984 Feb 1;217(3):595–599. doi: 10.1042/bj2170595. [DOI] [PMC free article] [PubMed] [Google Scholar]


