Abstract
Expression of the lytic cycle genes of Epstain-Barr virus (EBV) is induced in type I Burkitt's lymphoma-derived cells by treatment with phorbol esters (e.g., phorbol myristate acetate [PMA]), anti-immunoglobulin, or the cytokine transforming growth factor β (TGF-β). Concomitantly, all these agents induce apoptosis as judged by a sub-G1 fluorescence-activated cell sorter (FACS) profile, proteolytic cleavage of poly(ADP-ribose) polymerase (PARP) and terminal deoxynucleotidyltransferase-mediated dUTP-biotin nick end labeling (TUNEL) staining. However, caspase activation is not required for induction of the lytic cycle since the latter is not blocked by the caspase inhibitor ZVAD. Furthermore, not all agents that induce apoptosis in these cultures (for example, cisplatin and ceramide) induce the EBV lytic programme. Although it is closely associated with the lytic cycle, apoptosis is neither necessary nor sufficient for its activation. Multiparameter FACS analysis of cultures treated with PMA, anti-Ig, or TGF-β revealed BZLF1-expressing cells distributed in different phases of the cell cycle according to which inducer was used. However, BZLF1-positive cells did not appear to undergo apoptosis and accumulate with a sub-G1 DNA content, irrespective of the inducer used. This result, which suggests that lytic gene expression is protective, was confirmed and extended by immunofluorescence staining doubled with TUNEL analysis. BZLF1- and also gp350-expressing cells were almost always shown to be negative for TUNEL staining. Similar experiments using EBV-positive and -negative subclones of Akata BL cells carrying an episomal BZLF1 reporter plasmid confirmed that protection from apoptosis was associated with the presence of the EBV genome. Finally, treatment with phosphonoacetic acid or acyclovir prior to induction with PMA, anti-Ig, or TGF-β blocked the protective effect in Mutu-I cells. These data suggest that a late gene product(s) may be particularly important for protection against caspase activity and cell death.
Epstein-Barr virus (EBV) is carried by more than 90% of the adult population worldwide as a largely nonpathogenic infection. Primary infection, which is generally silent—but in adolescence may be associated with infectious mononucleosis (IM)—occurs through salivary exchange in the oropharynx (reviewed in reference 37). Whether or not the initial infection was symptomatic, the virus subsequently persists in healthy hosts for the rest of their life as a latent infection of resting memory B cells in the peripheral blood. In this population of cells, transcription of the viral genome is extremely restricted and may even be completely absent (2, 3, 34). Periodically, in most asymptomatic carriers of EBV, the virus is replicated and infectious virions can be recovered in oral secretions. This replication that results in the production of infectious virus is referred to as the EBV lytic cycle or program. It is assumed that activation of the lytic program occurs in memory B cells recirculating through the lymphoid tissue associated with the oropharyngeal mucosa; however, the mechanism underlying this viral reactivation in vivo is not clearly understood (reviewed in reference 48).
Infection in vitro by EBV induces the continuous proliferation of resting human B cells. The resulting lymphoblastoid cell lines (LCLs), which have a phenotype resembling activated B blasts, express only nine latency-associated EBV proteins. There are six nuclear proteins (EBNA-1, EBNA-2, EBNA-3A, EBNA-3B, EBNA-3C, and leader protein (LP)] and three membrane proteins (LMP-1, LMP-2A, and LMP-2B). Together they activate quiescent B cells into the cell cycle, maintain continuous proliferation, maintain the viral genome in a latent episomal form, and probably prevent cells from undergoing terminal differentiation or apoptosis (reviewed in reference 26). In vivo this ability of the virus to drive B-cell proliferation is important because these LCL-like cells appear to retain the capacity to undergo differentiation in germinal centers and thus permit latent genomes to enter the memory cell pool (2, 3, 48). In addition to causing IM, EBV is associated with several B-cell tumors, including endemic Burkitt's lymphoma (BL). The pattern of EBV gene expression in biopsy-derived BL cells differs from that found in LCLs in that the only nuclear protein detected is EBNA-1 and the membrane proteins are not expressed. This more restricted form of latency has been called latency type I, and the form found in LCLs has been termed latency type III (26, 37, 39, 40). Since it is very difficult to induce appreciable numbers of type III LCLs to activate the EBV lytic program, cultured BL cells which retain the type I phenotype provide the best in vitro model available for studying the switch between latency and EBV lytic replication (25, 39, 43). A variety of different agents have been reported to increase the proportion of EBV-infected BL cells entering the lytic cycle in vitro. These range from highly pleiotropic agents such as phorbol 12-myristate 13-acetate (PMA), which activate protein kinase C, to more physiologically relevant stimuli such as the immunomodulatory cytokine transforming growth factor β (TGF-β) or antibodies that cross-link surface immunoglobulin (Ig) and mimic antigen binding (4, 10, 12, 45, 46, 49, 54).
The EBV lytic programme is initiated by the expression of the viral immediate-early gene BZLF1. The BZLF1 protein (also known as Z, ZEBRA, Zta, and EB1) is a DNA-binding, b-zip transcription factor which has partial amino acid homology to the cellular proto-oncogene product c-Fos (5, 6, 16, 28, 50). BZLF1 binds to target sequences in the promoters of the early genes of EBV and cooperates with the BRLF1 protein to activate the cascade of gene expression that results in viral DNA replication and virion production (8, 16, 17, 50). Transcripts containing the BZLF1 open reading frame (ORF) are derived from two promoters, Zp and upstream Rp. The distal Rp also transcribes the BRLF1 gene; therefore, products consist of 1-kb monocistronic or 3-kb bicistronic mRNAs (30). Control elements responsive to phorbol esters (e.g., PMA and tetradecanoyl phorbol acetate) and signals from cross-linked surface Ig have been mapped in the Zp promoter, but the mechanism by which TGF-β activates BZLF1 expression is unknown (11, 14, 18, 41; our unpublished data).
Lytic EBV gene expression includes at least two protein products that have been reported to inhibit the process of programmed cell death (apoptosis). The BHRF1 protein is an early-gene product and appears to be a homolog of the cellular antiapoptotic protein Bcl2 (9, 21, 31, 36, 47). The product of BALF1 also has weak homology to Bcl2 and binds to Bax and Bak, and RNA containing the BALF1 ORF is also expressed in the early lytic programme (15, 32). Several reports suggest that in addition to potentially modifying apoptotic responses in infected cells, EBV lytic proteins may modulate cell cycle progression. When expressed in isolation, both the BZLF1 and BRLF1 proteins can modify cell cycle regulation: BZLF1 can induce cell cycle arrest, whereas BRLF1 has been shown to induce cellular DNA synthesis (38, 44). The aim of this study was therefore to investigate the relationship between apoptosis and activation of the EBV lytic program and also to take the opportunity to determine whether coordinated lytic gene expression significantly affects cell cycle regulation in infected BL cells.
MATERIALS AND METHODS
Cell culture.
The Mutu-I BL cell line was cultured in RPMI 1640 medium supplemented with penicillin, streptomycin, glutamine (all from Gibco-BRL), and 10% Serum Supreme (BioWhittaker, Wokingham, United Kingdom). The Akata 6 (EBV-positive) and the Akata 31 (EBV-negative) cell lines were stably transfected with the plasmid p294:Zp-552Xwt (25, 46). These were cultured in RPMI 1640 medium supplemented with penicillin, streptomycin, glutamine, and 10% fetal calf serum (Gibco-BRL). p294:Zp-552Xwt is an episomal vector carrying the BZLF1 ORF under the control of the BZLF1 promoter (25). All cell lines were maintained at 37°C in a 10% CO2 incubator. The cells were routinely fed at a dilution of 1:4; for experimental analysis, cells were diluted to 3 × 105/ml 24 h prior to manipulation.
Antibodies and reagents used in the induction of the lytic cycle and apoptosis.
Sheep anti-mouse Ig conjugated to horseradish peroxidase (HRP) (Amersham, Little Chalfont, United Kingdom), goat anti-rabbit HRP-conjugated Ig, goat anti-human HRP-conjugated Ig, goat anti-mouse IgG conjugated to fluorescein isothiocyanate (FITC) (Dako A/S), rabbit anti-mouse IgG conjugated to tetramethylrhodamine isothiocyanate (TRITC) (Sigma, Poole, United Kingdom), and anti-poly(ADP ribose) polymerase (PARP) polyclonal antibody (Boehringer Mannheim, Lewes, United Kingdom) were all used as recommended by the manufacturers. The human serum EE (40) was used at a dilution of 1:10,000 in Western blotting experiments. This serum is from a chronic IM patient with high levels of antibodies to EBV lytic-cycle antigens. It had the following initial reciprocal titers; VCA, 80,000; EA, 20,000; EBNA, 64. Most of the proteins it identifies in Western blots are EBV early antigens. The anti-BZLF1 monoclonal antibody (MAb) BZ-1 (53) hybridoma supernatant was used undiluted for FACS analysis and 1:2 for immunofluorescence. The 72A1 anti-gp350 MAb (22) was used at a concentration of 1:100, the anti-BHRF1 MAb (36) was used at a concentration of 1:100, and the EBV.OT41A anti-BDRF1/VCA-p40 MAb was used at a concentration of 1:1,000 (51). Cisplatin (David Bull Laboratories) and ceramide (Sigma) were used at final concentrations of 10 μg/ml and 250 μM, respectively. The panspecific caspase inhibitor benzyloxycarbonyl-Val-Ala-Asp(Ome)-fluoromethylketone (ZVAD-fmk) was used at a final concentration of 100 μM (Enzyme Systems Products, Livermore, Calif.). Affinity-purified goat anti-human IgM (μ-chain specific; Sigma) was resuspended in 0.135 M sodium chloride at a concentration of 1 mg/ml (100× final concentration). Human recombinant TGF-β1 rTGF-β1 (R&D Systems, Minneapolis, Minn.) was rehydrated in 4 mM HCl–1 mg of bovine serum albumin per ml solution at a concentration of 2 μg/ml and used at a final concentration of 5 ng/ml in all experiments. PMA (Sigma) was dissolved in dimethyl sulfoxide and used at a final concentration of 30 ng/ml. Rabbit anti-human IgG (Dako) was used at a final concentration of 5 μg/ml. Lytic-cycle inductions were performed by treating with TGF-β1, anti-IgM, anti-IgG, or PMA for 24 to 48 h as appropriate. In some instances, cells were pretreated with 120 μg of phosphonoacetic acid (PAA) (Sigma) per ml or 100 μg of acyclovir (Zurich Pharmaceuticals, London, United Kingdom) per ml for 1 h prior to addition of the inducing agent.
Protein content estimation and Western blotting.
Cells were lysed in RIPA lysis buffer (50 mM Tris [pH 8], 150 mM NaCl, 1% Nonidet P-40, 0.5% sodium deoxycholate, 0.1% sodium dodecyl sulfate [SDS]) supplemented with 1 mM phenylmethylsulfonyl fluoride (Sigma) and complete protease inhibitor cocktail (Boehringer Mannheim). The protein concentration was estimated spectrophotometrically at 750 nm in a Lambda Bio UV/Vis spectrometer (Perkin-Elmer, Norwalk, Conn.) using the Bio-Rad detergent-compatible assay, exactly as described by the manufacturer (Bio-Rad, Hemel Hempsted, United Kingdom). Protein was diluted to a concentration of 2 mg/ml and further diluted in an equal volume of 2X SDS protein sample buffer (60 mM Tris [pH 6.8], 2% [wt/vol] SDS, 20% [vol/vol] glycerol, 2% [vol/vol] 2-mercaptoethanol, bromophenol blue), and 50 to 100 μg was loaded onto 7.5 or 10% polyacrylamide gels for SDS-polyacrylamide gel electrophoresis. The Western blotting process was performed as previously described (52), and proteins were visualized by enhanced chemiluminescence (Amersham), as described by the manufacturer. Autoradiograms were then scanned and processed using a UMAX PowerLook III scanner and Adobe Photoshop software (Adobe Systems).
Cell cycle analysis.
Cell cycle analysis was performed by flow cytometry. Cells were harvested by centrifugation, washed in ice-cold phosphate-buffered saline, and fixed in 80% ethanol that had been prechilled to −20°C. Fixed cells were stored at 4°C for up to 1 week. They were then repelleted and resuspended at a concentration of ∼106/ml in PBS containing 18 μg of propidium iodide (PI; Sigma) per ml and 8 μg of RNase A (Sigma) per ml (PI solution). After the cells were incubated in the dark for at least 1 h, cell cycle profile analysis was performed on 10,000 to 20,000 cells with a FACSort flow cytometer using the Cellquest analysis program (Becton Dickinson).
BZLF1/cell cycle staining.
The cell cycle distribution of BZLF1-positive cells was assessed essentially as previously described for analyzing EBNA3C positive cells (35). Briefly, total cells were harvested by centrifugation, fixed in methanol at −20°C, and rehydrated in cold PBS for at least 1 h. The cells were stained by resuspension in BZ-1 hybridoma supernatant for 1 h at room temperature. They were then washed twice in PBS and resuspended in goat anti-mouse FITC-conjugated antibody for 30 min at room temperature. Following three further washes in PBS, the cells were resuspended in PI solution for at least 1 h and then analyzed by flow cytometry. Total populations were initially gated to remove doublets and cell debris, and, where appropriate, the BZLF1-positive population was gated for high FITC compared to uninduced cells stained in exactly the same manner.
BZLF1-TUNEL/gp350-TUNEL double staining.
In situ terminal deoxynucleotidyltransferase-mediated dUTP-biotin nick end labelling (TUNEL) analysis was performed using an FITC in situ cell death detection kit (Boehringer Manheim). TUNEL and immunostaining was performed essentially as previously described (52). Briefly, 106 cells were harvested from appropriate cultures following 48 h of lytic-cycle induction. The cells were washed in PBS, and cytospins of 8 × 104 cells were made. After being air dried, the cells were fixed in methanol-acetone (1:1) for 20 min at −20°C. After rehydration in PBS for 10 min, the cells were permeabilized with 100 μl of 0.1% (vol/vol) Triton X-100 in 0.1% sodium citrate for 5 min on ice. The cells were then incubated in a humidified chamber at 37°C for 90 min at a ratio of enzyme to FITC label solution as instructed by the manufacturer. Following two washes in PBS, cytospins were incubated with 20% normal rabbit serum in PBS for 30 min at room temperature. The cytospins were then washed twice in PBS and incubated in BZ-1 hybridoma supernatant diluted 1:2 in 20% normal rabbit serum–PBS or anti-gp350 MAb as appropriate for 1 h at room temperature. They were then washed twice in PBS and incubated for 1 h at room temperature with rabbit anti-mouse IgG conjugated to TRITC (diluted 1:128 in 20% normal rabbit serum–PBS). They were washed three times in PBS, mounted in Citifluor, and visualized for TUNEL and antigen positivity on a Zeiss Axiovert 100M confocal imaging microscope using excitation wavelengths of 488 and 543 nm, respectively. Images were processed using Zeiss LSM510 software.
RESULTS
Agents that induce the EBV lytic programme also induce apoptosis.
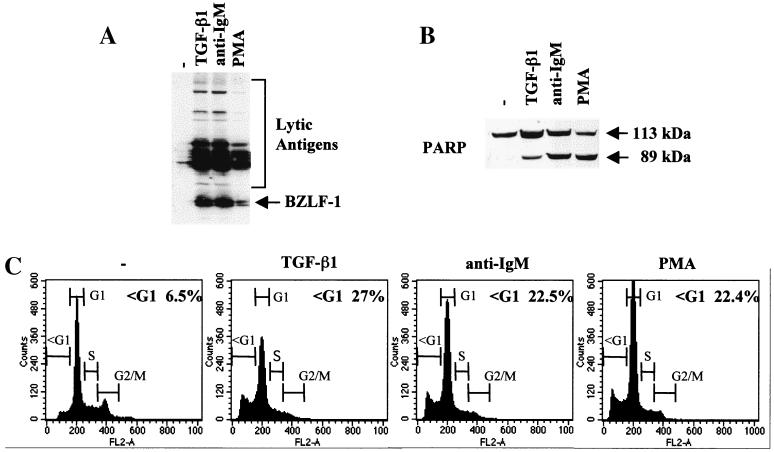
TGF-β is a pleiotropic cytokine which can exert a variety of effects on cell proliferation, differentiation, and apoptosis (reviewed in reference 33). We and others recently showed that it induced apoptosis in the majority of BL-derived cell lines investigated (7, 23, 24, 29); our unpublished data). We also observed that in several EBV-carrying BL cell lines exhibiting a type I pattern of latency, TGF-β induced expression of the EBV immediate-early and early antigens characteristic of the lytic programme (Fig. 1A and our unpublished data). This was consistent with earlier reports that implicated TGF-β in the lytic replication of EBV (4, 12). Since activation of the lytic cycle paralleled activation of the apoptosis programme, further analyses were performed using the Mutu-I BL cell line to determine whether other agents that trigger the lytic switch also induce apoptosis. After treatment of Mutu-I cells with antibody directed against cell surface Ig (in this case anti-IgM) or the tumor-promoting phorbol ester PMA, in addition to expressing BZLF1 and various early antigens (Fig. 1A) the treated Mutu-I cells exhibited several features characteristic of apoptosis. Western blotting showed cleavage of PARP, and flow cytometry revealed a PI-stained population with an apparently sub-G1 (less than 2N) DNA content (Fig. 1B and C) TUNEL analysis confirmed the cells were undergoing apoptosis (data not shown; see Fig. 4 and 5).
FIG. 1.
Inducers of the lytic cycle of EBV concomitantly induce apoptosis. (A and B) Western blot analysis of 50 μg of total cellular RIPA lysates prepared from Mutu-I cells, untreated (−) or treated for 48 h with TGF-β1, anti-IgM, or PMA as described in Materials and Methods. (A) Probed with EE human serum; (B) probed with a polyclonal anti-PARP rabbit serum. (C) Cell cycle distribution of samples taken from the same experiment. Cells were fixed, stained for DNA content with PI, and analyzed by flow cytometry.
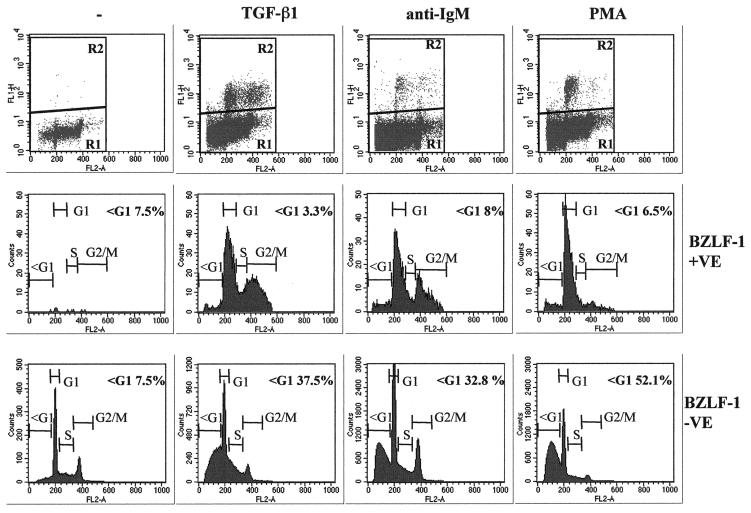
FIG. 4.
Most BZLF1-expressing cells have a ≥2N DNA content. Control untreated (−) Mutu-I cells or those treated with TGF-β, anti-IgM, or PMA for 48 h were fixed and stained with the BZ-1 MAb and then stained for DNA content with PI. The top panels show flow cytometry plots of fluorescence (FL1-H) against PI content (FL2-A). The bottom two series of panels show analyses of the DNA content of BZLF1-fluorescence-positive (R2) and fluorescence-negative (R1) cells gated as shown in the top panels.
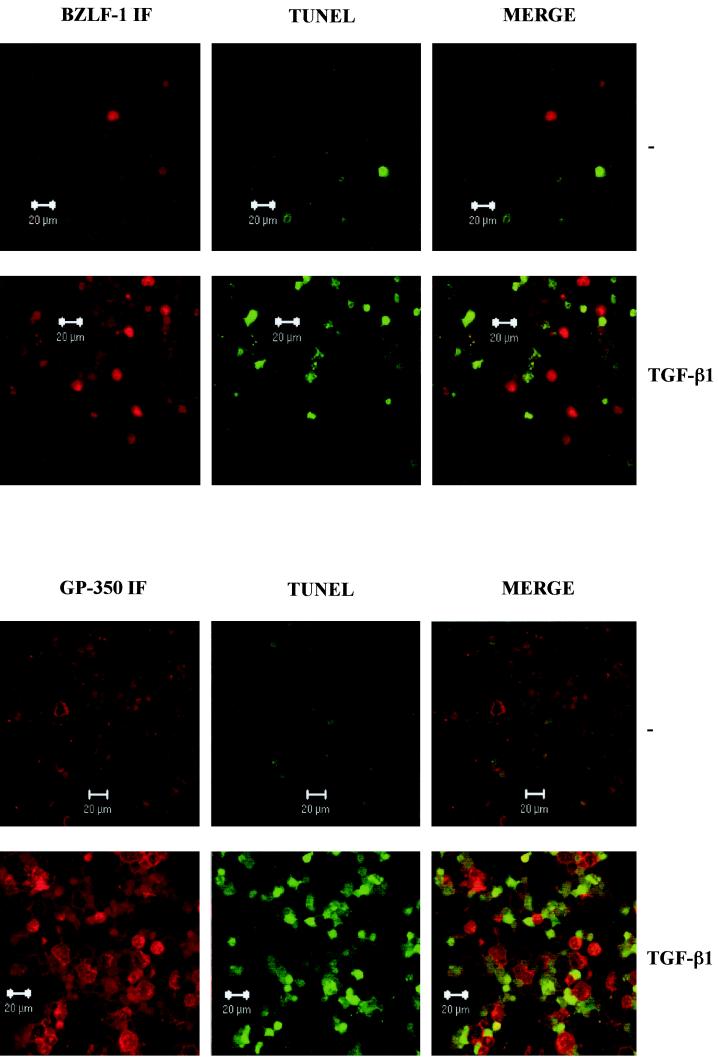
FIG. 5.
Cells expressing lytic EBV antigens and TUNEL-positive cells are mutually exclusive. Control untreated (−) and TGF-β-treated samples of Mutu-I cells were analyzed for BZLF1 immunofluorescence and TUNEL staining (A) and gp350 immunofluorescence and TUNEL staining (B) by confocal microscopy. The merged images show that BZLF1- and gp350-expressing cells are nearly all TUNEL negative.
Caspase activation and apoptosis are neither necessary nor sufficient for activation of the lytic program.
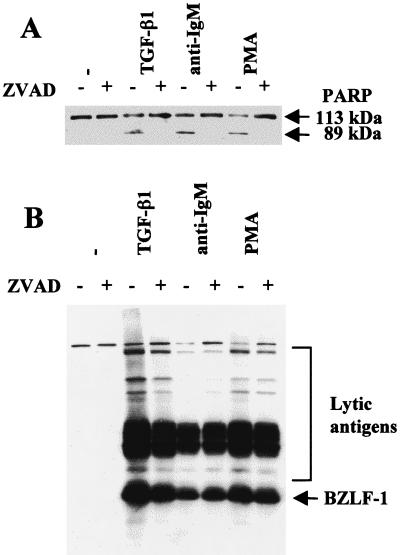
Since activation of the lytic cycle correlated with apoptosis, we determined whether the caspase activation which is generally central to the execution of apoptosis was necessary for the switch from latency to viral lytic replication. Experiments were performed using the panspecific inhibitor of caspase activity ZVAD. This peptide effectively blocked the proteolytic cleavage of PARP in TGF-β-, anti-IgM-, and PMA-treated Mutu-I cells but had little or no effect on the induction of BZLF1 and EBV early antigens (Fig. 2).
FIG. 2.
Inhibition of caspase activity does not block induction of the lytic cycle. Mutu-I cells were pretreated with the pan-caspase inhibitor ZVAD for 1 h prior to induction of the lytic cycle by TGF-β, anti-IgM, or PMA. Apoptosis (A) and induction of lytic antigens (B) were assayed by Western blotting and probing with an anti-PARP rabbit serum or serum EE, respectively. ZVAD alone had no obvious effect in the untreated cells (−) (first two tracks in both panels).
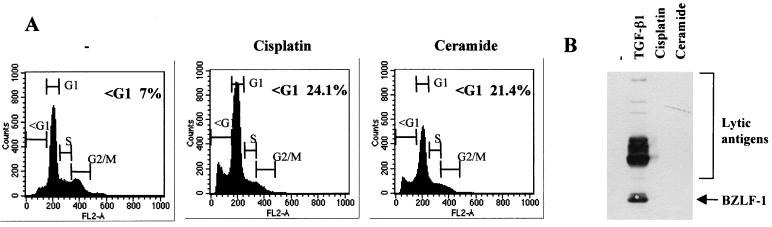
Although apoptosis (or at least caspase activity) was not necessary for the induction of the lytic cycle, we went on to determine whether apoptosis induced by alternative pathways—such as those signaled by DNA damage or ceramide—was associated with the activation of viral replication. Although both agents rapidly induced a significant level of apoptosis as judged by flow cytometry and PARP cleavage (Fig. 3A and our unpublished data), neither the genotoxin cisplatin nor ceramide activated the expression of BZLF1 or the complex of early antigens (Fig. 3B). We concluded from these experiments that at least three molecular pathways which trigger apoptosis in BL cells (consitutively active protein kinase C, TGF-β signaling, and signaling from surface Ig) also activate transcription of the lytic switch BZLF1 but that apoptosis per se is neither sufficient nor necessary to activate the lytic program.
FIG. 3.
Induction of apoptosis per se does not induce the EBV lytic cycle. Mutu-I cells were treated with cisplatin or ceramide for 24 h or left untreated (−). Apoptosis and the induction of lytic-cycle antigens were assessed by flow cytometry (A) and Western blotting and probing with EE serum (B).
Cells expressing BZLF1 did not undergo apoptosis and were distributed throughout the cell cycle according to the nature of the agent used for induction.
Expression of BZLF1 is the earliest marker of EBV-infected cells entering the lytic cycle, and the availability of suitable anti-BZLF1 monoclonal antibodies made it possible to identify individual BZLF1-positive cells by flow cytometry. To analyze single cells in a mixed population, Mutu-I cells that had been induced into the lytic cycle were immunostained for BZLF1 expression and costained with PI for determination of the DNA content. These were then subjected to two-dimensional flow cytometric analysis that identified the BZLF1-expressing cells and visualized their distribution in the cell cycle. Electronic gating allowed a comparison of the cell cycle distribution of BZLF1-positive and BZLF1-negative cells (Fig. 4). Inspection of the bulk BZLF1-negative populations (R1 and bottom panels) before and after treatment confirmed that TGF-β, anti-IgM, and PMA all induce a significant number of apoptotic Mutu-1 cells (sub-G1 populations of 37.5, 32.8, and 52.1%, respectively). In contrast, the populations expressing detectable levels of BZLF1 (R2 and middle panels) included very few apoptotic cells distributed to the left of the G1 peak (3.3, 8, and 6.5%, compared with 7.5% in the untreated control cells). This was consistent with these cells being protected from programmed cell death.
A comparison of the cell cycle profiles after the three different treatments also suggests that the BZLF1-expressing cells respond differently depending on the stimulus used. After TGF-β or anti-IgM treatment, the BZLF1-positive cells were clearly distributed throughout the cell cycle; however, when the cells were treated with PMA, there was an accumulation of 2N cells consistent with a G1 arrest. Confirmation that after induction the BZLF1-positive and apoptotic populations were mutually exclusive was obtained by using a combination of BZLF1 immunofluorescence coupled with TUNEL staining for the products of endonuclease cleavage of cellular DNA. Analysis by confocal microscopy in several different experiments consistently showed that no BZLF1-expressing cells were also TUNEL positive. Figure 5A shows the results of a representative experiment using TGF-β. PMA and anti-IgM produced very similar results (data not shown). Since a possible explanation of this result was that apoptosis resulted in the loss of the BZLF1 epitope, a similar experiment was performed utilizing the late virion protein gp-350 as a marker of the lytic program; this antigen is expressed at the cell surface. A representative experiment, again using TGF-β, is illustrated in Fig. 5B. As before, PMA and anti-IgM produced results that were largely indistinguishable from these results (data not shown). gp350 staining and TUNEL were almost always mutually exclusive. These data are also consistent with the hypothesis that lytic EBV gene expression blocks or very significantly delays apoptosis in BL cells.
Protection from apoptosis is dependent on the presence of EBV.
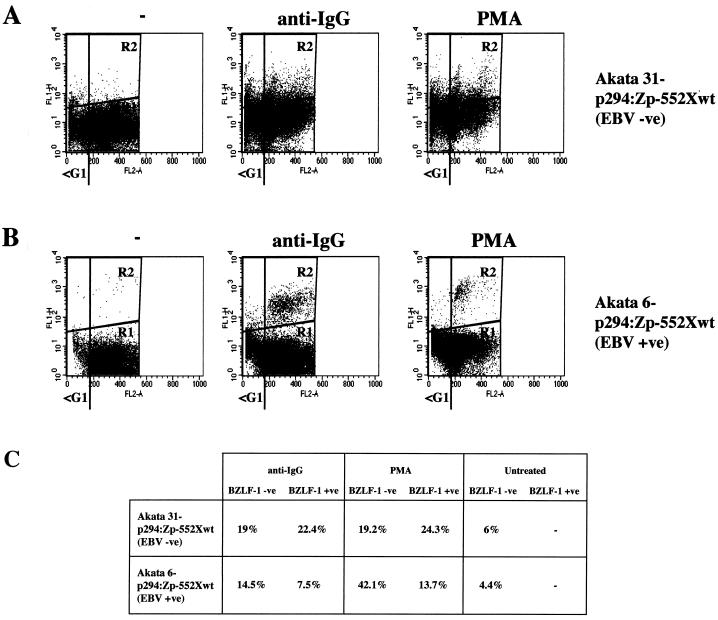
It was unclear from these experiments whether the apparent protection seen in the BZLF1-expressing cells was due to an EBV gene product (other than BZLF1). Also we were unable to rule out the possibility that only cells which were resistant to apoptosis activate the BZLF1 gene and successfully initiate the EBV lytic program.
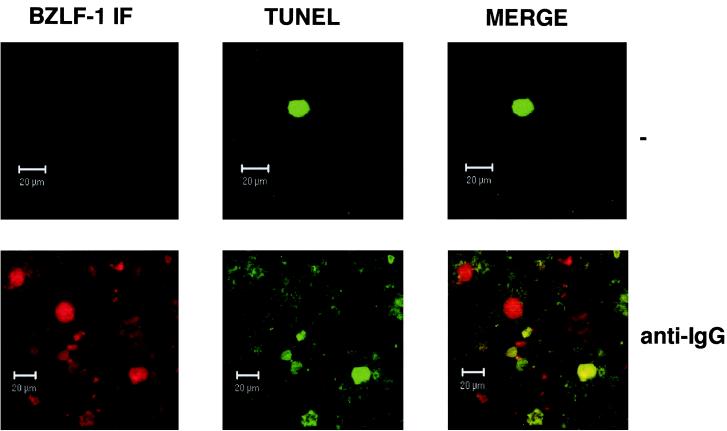
To address these points, use was made of clones from the Akata BL cell line which either retain EBV episomes or have lost them to become EBV negative (25, 42). Such clones were transfected to produce stable subclones carrying episomal reporter plasmids containing the BZLF1 coding region under control of the BZLF1 promoter (p294:Zp-552Xwt). The BZLF1 gene in this system has been shown to reconstitute the normal regulation of Zp and to express BZLF1 protein at similar levels to the whole EBV genome (25). EBV-negative Akata 31-p294:Zp-552Xwt and EBV-positive Akata 6-p294:Zp-552Xwt cells were treated with cross-linking anti-Ig (in this case anti-IgG) or PMA and were then assayed as described above, using the MAb to BZLF1 doubled with PI staining. Results of a representative experiment are shown in Fig. 6. Flow cytometry revealed that the BZLF1-positive and -negative populations were largely indistinguishable for the Akata 31-p294:Zp-552Xwt cells (Fig. 6A and C); both had similar percentages of cells in the sub-G1 compartment (22.4 and 19% for anti-Ig; 24.3 and 19.2% for PMA). In contrast, for the Akata 6-p294:Zp-552Xwt cells (Fig. 6B and C), the BZLF1-positive cells were excluded from the apoptotic, sub-G1 population (7.5% relative to 14.5% for anti-IgG; 13.7% relative to 42.1% for PMA). This latter result was very similar to that obtained with the EBV-positive Mutu-I cells (Fig. 4). These data strongly suggested that protection from apoptosis was dependent on the presence of the EBV genome, since sub-G1, BZLF-1-positive cells were seen only in an EBV-negative background (Fig. 6A). Consistent with this, confocal microscopy revealed that TUNEL-positive and BZLF1-positive cells were mutually exclusive in the Akata 6-p294:Zp-552Xwt cells (data not shown) but double staining could be seen in Akata 31-p294:Zp-552Xwt cells (Fig. 7).
FIG. 6.
Protection from apoptosis is EBV dependent. (A and B) Akata 31-p294:Zp-552Xwt (EBV −ve) (A) and Akata 6-p294:Zp-552Xwt (EBV +ve) (B) cells were treated with carrier (−), anti-IgG, or PMA for 48 h and then stained for BZLF1 and stained for DNA content with PI. Flow cytometry was then performed essentially as described in the legend to Fig. 4. (C) Percentages of BZLF1-negative and BZLF1-positive cells with an apoptotic, sub-G1 distribution.
FIG. 7.
BZLF1 and TUNEL staining overlap in some Akata 31-p294:Zp-552Xwt cells. Control untreated (−) and anti-IgG-treated samples of Akata 31-p294:Zp-552Xwt cells were analyzed for BZLF1 immunofluorescence and TUNEL staining by confocal microscopy. Merged images show that BZLF1- and TUNEL-positive cells are not always mutually exclusive.
Inhibitors of EBV lytic DNA replication and late-gene expression block the survival of cells that express BZLF1 after treatment with PMA, TGF-β, or anti-IgM.
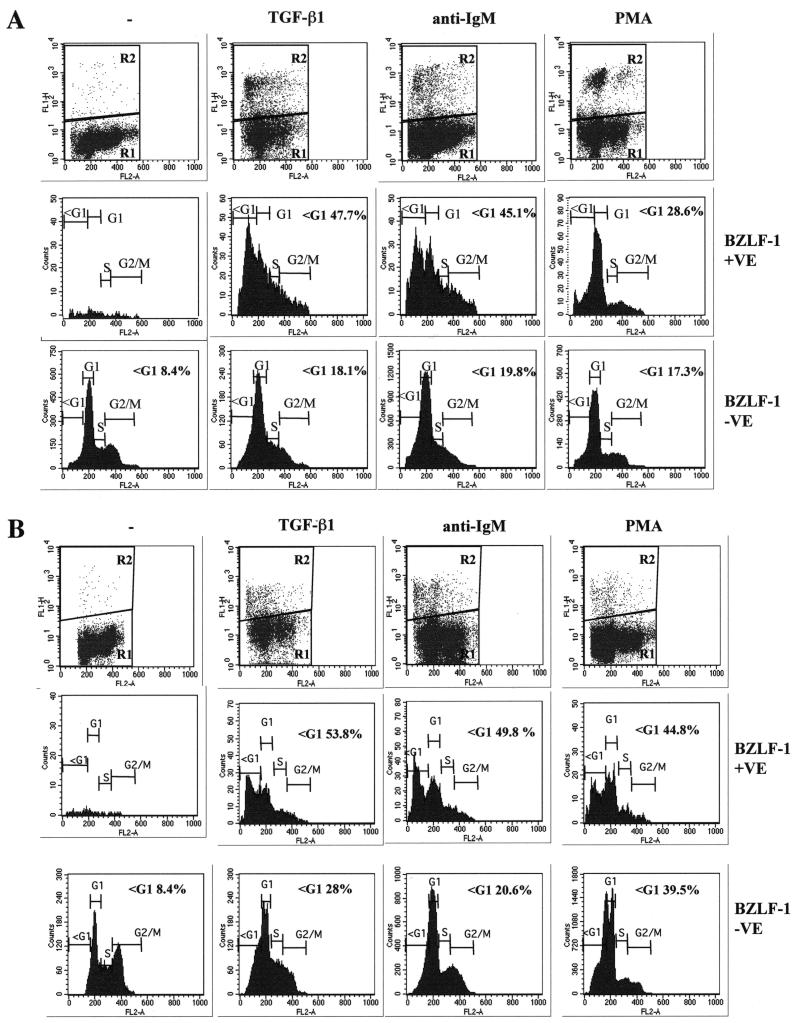
The results obtained with Akata presented in the preceding section were consistent with one or more EBV lytic gene product inhibiting apoptosis in the Mutu-I cells treated with each of three different apoptogenic agents. To help focus on which product(s) might contribute to this effect, a series of experiments were performed with agents which have a minimal effect on immediate-early and early EBV gene expression but which block viral DNA synthesis and late-gene expression. In EBV biology, these agents define the early and late proteins of the lytic cycle (26, 37).
Initially, similar experiments to those illustrated in Fig. 4 were performed, but prior to the addition of the inducing agent (TGF-β, anti-IgM, or PMA) the Mutu-1 cells were pretreated for 1 h with the inhibitor of viral DNA synthesis PAA (27). A comparison of these results (Fig. 8A) with those in Fig. 4 shows clearly that in the PAA-treated population, when BZLF1-positive cells were identified they included a significant proportion of cells (47.7, 45.1, and 28.6%) distributed in the sub-G1 apoptotic compartment. This was in contrast to the cells that were not treated with PAA and that had a BZLF1-positive population largely protected from apoptosis (Fig. 4). To show that the loss of protection was not a specific effect of PAA on the virus or cell, similar experiments were performed using acyclovir. This also blocks EBV DNA replication but by a biochemically distinct mechanism (27). Acyclovir treatment produced results that were almost indistinguishable from those produced by PAA; that is, it also inhibited the antiapoptotic effect (compare Fig. 8A and B). We concluded from these experiments that the protection against apoptosis afforded by EBV depends largely on one or a combination of late viral gene products. Concomitantly, these experiments conclusively demonstrated that sub-G1 apoptotic cells do not lose the BZLF1 epitope.
FIG. 8.
Inhibitors of EBV DNA replication and late-gene expression block the protection against apoptosis. (A) Mutu-I cells were pretreated for 1 h with PAA, treated with TGF-β, anti-IgM, or PMA for 48 h, and then stained for BZLF1 and DNA content. Flow cytometry was performed as described in the legend to Fig. 4 and Materials and Methods. (B) Mutu-I cells were pretreated for 1 hour with acyclovir and then treated and analyzed as in panel A.
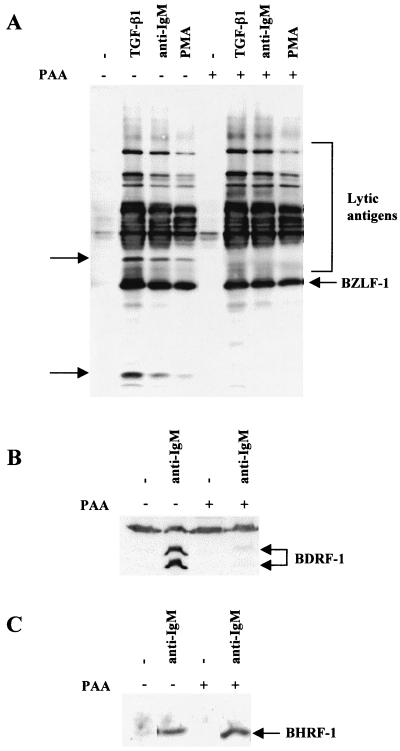
Western blot analysis showed that PAA pretreatment blocked the expression of at least two unidentified lytic antigens recognized by the EE human serum and confirmed that most of the proteins recognized by EE on Western blots are early antigens (Fig. 9A). To confirm that PAA had specifically blocked late-gene expression, lysates were Western blotted for the BDRF1/VCA-p40 late antigen (51). PAA almost completely blocked the induction of this antigen by anti-IgM treatment (Fig. 9B). However, PAA did not significantly alter the expression of the EBV Bcl2 homologue BHRF1 after anti-IgM treatment (Fig. 9C).
FIG. 9.
PAA treatment blocks the induction of lytic viral antigens but not the induction of BHRF1. Mutu-I cells were pretreated for 1 h with PAA and then treated for 48 h with the inducing agents indicated. (A) Western blot analysis of total cellular RIPA lysates was performed with EE serum. The lytic antigens whose expression is blocked by PAA are indicated by arrows. (B) Lysates from the induction with anti-IgM with or without PAA pretreatment were blotted and probed for the expression of BDRF1/VCA.p40 late antigen. The doublet corresponding to BDRF1 is indicated, and the nonspecific band recognized by the antibody shows equal loading of protein in each track. (C) Similar lysates to those in panel B were blotted and probed for BHRF1.
DISCUSSION
Since it was discovered that EBV encodes at least one lytic protein (BHRF1) which is related to Bcl2 and exhibits antiapoptotic activity, there has been a great deal of speculation that during the lytic cycle EBV may actively block the cell death program (1, 21, 26, 32, 37, 47). Various experiments have been reported that suggest that BHRF1, BALF1, and the latent protein LMP1 can suppress apoptosis. However, in most instances single isolated genes were expressed (or overexpressed) from transfected plasmids (21, 32, 47). Similarly, analyses concerned with the behavior of cells in response to the immediate-early transactivator proteins BZLF1 and BRLF1 have generally utilized similar single-gene approaches (38, 44). Here we have attempted to investigate what happens to BL cells during the reactivation of latent EBV genomes. In this situation the individual viral proteins are expressed in B cells and function in the context of the coordinated pattern of EBV gene expression, which ultimately results in de novo synthesis and assembly of infectious virions.
We showed that, at least in group I BL cells, activation of the EBV lytic cycle is closely associated with programmed cell death; it is possible that these processes may also be linked in vivo. Three widely used treatments that induce the lytic cycle also induce apoptosis in these cells. Since at least two of these, the cytokine TGF-β and the cross-linking of surface Ig (to mimic antigen binding), activate physiologically relevant pathways, we suggest that apoptosis probably accompanies the reactivation of EBV from some latently infected cells in vivo. BL cells phenotypically resemble germinal-center centroblasts, which are very prone to apoptosis in vivo as well as in vitro (19, 20). It is not unreasonable to suggest, therefore, that EBV reactivation may commonly accompany apoptosis in mucosa-associated lymphoid tissue such as the tonsils.
Since apoptosis involves the activation of numerous proteolytic enzymes (the caspases) and at least one endonuclease (caspase-activated DNase) (reviewed in reference 13), it is also reasonable to hypothesize that EBV might have evolved mechanisms to suppresss this process, or at least to inhibit the enzymes which could destroy both viral proteins and DNA. Our analyses of the cells that entered the lytic cycle (operationally defined as those which express a detectable level of BZLF1) are consistent with this hypothesis. We found that the BZLF1-positive cells showed no evidence of chromatin condensation or DNA cleavage that is associated with apoptosis; they appeared completely protected. This antiapoptotic activity was not provided by BZLF1 alone, since expression of BZLF1 in the EBV-negative Akata 31 cells produced no protective effect. The activated EBV genome, present in Akata 6, was necessary to prevent cell death. These observations are consistent with the notion that the early lytic proteins BHRF1 and BALF1 contribute to the inhibition of apoptosis. However, further experiments showed that, alone, these two proteins are probably inadequate for full protection. The surprising result we obtained was that if drugs were added which block lytic EBV DNA synthesis and therefore also the expression of genes operationally defined as late, the survival of BZLF1-positive cells was abrogated. Accepting the caveat that both PAA and acyclovir might have unknown effects on the expression and/or function of critical viral or cell proteins, these data lead us to the conclusion that EBV DNA replication or a late EBV gene product is an absolute requirement for the effective protection of infected cells from apoptotic death. This may be a direct antiapoptotic activity, or it could be indirect. For instance, although the level of BHRF1 expression was unaltered by pretreatment with PAA, we cannot exclude the possibility that BHRF1 requires posttranslational modification by a late-gene product for full activity. Inspection of the predicted amino acid sequences encoded by the 27 mapped late genes (15) did not suggest any obvious candidate proteins. An analysis of recombinant viruses in which each late gene is systematically deleted or mutated may be the only approach that will identify the late antiapoptotic function(s) of EBV.
When the cells carrying EBV that had entered the lytic cycle (BZLF1-positive cells) were examined by flow cytometry for their position in the cell cycle, BZLF1-expressing cells could be seen in all of the phases of the cell cycle. However, the relative distributions appeared to be determined by the treatment used to activate the lytic program rather than by the expression of viral proteins. Thus, it appears that changes in the cell cycle profile, such as the G1 arrest in PMA-treated cells, are dominant over any cell cycle effects produced by individual EBV lytic gene products.
In summary, we showed here that various agents that induce the EBV lytic cycle, including at least two which may well be important during reactivation of the virus in vivo, have the capacity to activate the apoptosis program in B cells. However, apoptosis is neither necessary nor sufficient for reactivation. Furthermore, we showed that in cells treated with these agents and expressing the full spectrum of EBV late lytic antigens, apoptosis is almost completely inhibited or significantly delayed. This is, to our knowledge, the first direct demonstration that EBV can modulate the regulation of programmed cell death during the reactivation of the latent genome and production of new virions.
ACKNOWLEDGMENTS
We thank Andy Morgan (Bristol) for supplying the gp350 MAb, Jap Middeldorp (Amsterdam) for providing the EBV.OT41A MAb, and Alan Rickinson (Birmingham) for providing the EE serum.
We are very grateful to the Wellcome Trust for supporting this research through project grants 047383 and 0050096 to M.J.A.
REFERENCES
- 1.Allday M J. Apoptosis and Epstein-Barr virus in B cells. Epstein-Barr Rep. 1996;3:55–65. [Google Scholar]
- 2.Babcock G J, Decker L L, Volk M, Thorley-Lawson D A. EBV persistence in memory B cells in vivo. Immunity. 1998;9:395–404. doi: 10.1016/s1074-7613(00)80622-6. [DOI] [PubMed] [Google Scholar]
- 3.Babcock G J, Decker L L, Freeman R B, Thorley-Lawson D A. Epstein-Barr virus-infected resting memory B cells, not proliferating lymphoblasts, accumulate in the peripheral blood of immunsuppressed patients. J Exp Med. 1999;190:567–576. doi: 10.1084/jem.190.4.567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bauer G, Hofler P, Simon M. Epstein-Barr virus induction by a serum factor. Characterisation of the purified factor and the mechanism of its activation. J Biol Chem. 1982;257:1141–11415. [PubMed] [Google Scholar]
- 5.Countryman J, Miller G. Activation of expression of latent Epstein-Barr herpesvirus after gene transfer with a small cloned sub-fragment of heterogeneous viral DNA. Proc Natl Acad Sci USA. 1985;82:4085–4089. doi: 10.1073/pnas.82.12.4085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chang Y N, Dong D L Y, Hayward G S, Hayward D. The Epstein-Barr virus Zta transactivator: a member of the bZip family with unique DNA-binding specificity and a dimerization domain that lacks the characteristic heptad leucine zipper motif. J Virol. 1990;64:3358–3369. doi: 10.1128/jvi.64.7.3358-3369.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chaouchi N, Arvanitakis L, Auffredou M T, Blanchard D A, Vasquez A, Sharma S. Characterization of transforming growth factor beta-1 induced apoptosis in normal human B cells B lymphoma cell lines. Oncogene. 1995;11:1615–1622. [PubMed] [Google Scholar]
- 8.Chevallier-Greco A, Manet E, Chavrier P, Mosnier C, Daillie J, Sergeant A. Both Epstein-Barr virus (EBV)-encoded transactivating factors, EB1 and EB2, are required to activate transcription from an EBV early promoter. EMBO J. 1986;5:2343–3249. doi: 10.1002/j.1460-2075.1986.tb04635.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cleary M L, Smith S, Sklar J. Cloning and structural analysis of cDNAs for bcl2 and a hybrid bcl2/immunoglobulin transcript resulting from the t14;18 translocation. Cell. 1986;74:609–619. doi: 10.1016/0092-8674(86)90362-4. [DOI] [PubMed] [Google Scholar]
- 10.Davies A H, Grand R J A, Evans F J, Rickinson A B. Induction of Epstein-Barr virus lytic cycle by tumor-promoting and non-tumor-promoting phorbol esters requires active protein kinase C. J Virol. 1991;65:6838–6844. doi: 10.1128/jvi.65.12.6838-6844.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Diabata M, Speck S H, Mulder C, Sairenji T. Regulation of the BZLF1 promoter of Epstein-Barr virus by second messengers in anti-immunoglobulin treated B cells. Virology. 1994;198:446–454. doi: 10.1006/viro.1994.1056. [DOI] [PubMed] [Google Scholar]
- 12.Di Renzo L, Altiok A, Klein G, Klein E. Endogenous TGF-beta contributes to the induction of the EBV lytic cycle in two Burkitt lymphoma lines. Int J Cancer. 1994;57:914–919. doi: 10.1002/ijc.2910570623. [DOI] [PubMed] [Google Scholar]
- 13.Earnshaw W C, Martins L M, Kaufmann S H. Mammalian caspases: structure, activation, substrates and functions during apoptosis. Annu Rev Biochem. 1999;68:383–424. doi: 10.1146/annurev.biochem.68.1.383. [DOI] [PubMed] [Google Scholar]
- 14.Fahmi H, Cochet C, Hmama Z, Opopon P, Joab I. Transforming growth factor beta 1 stimulates expression of the Epstein-Barr virus BZLF1 immediate-early gene product ZEBRA by an indirect mechanism which requires the MAPK kinase pathway. J Virol. 2000;74:5810–5818. doi: 10.1128/jvi.74.13.5810-5818.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Farrell P J. Epstein-Barr virus. In: O.'Brien S, editor. Genetic maps. Vol. 1. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1993. pp. 120–129. [Google Scholar]
- 16.Farrell P J, Rowe D T, Rooney C M, Kouzarides T. Epstein-Barr virus BZLF1 trans-activator specifically binds to a consensus AP-1 site and is related to c-fos. EMBO J. 1989;8:127–132. doi: 10.1002/j.1460-2075.1989.tb03356.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Flemington E, Speck S H. Autoregulation of Epstein-Barr virus putative lytic switch gene BZLF1. J Virol. 1990;64:1227–1232. doi: 10.1128/jvi.64.3.1227-1232.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Flemington E, Speck S H. Identification of phorbol ester response elements in the promoter of the Epstein-Barr virus putative lytic switch gene BZLF1. J Virol. 1990;64:1217–1226. doi: 10.1128/jvi.64.3.1217-1226.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gregory C D, Dive C, Henderson S, Smith C A, Williams G T, Gordon J, Rickinson A B. Activation of Epstein-Barr virus latent genes protects human B cells from death by apoptosis. Nature. 1991;349:612–614. doi: 10.1038/349612a0. [DOI] [PubMed] [Google Scholar]
- 20.Gregory C D, Tursz T, Edwards C F, Tetaud C, Talbot M, Caillou B, Rickinson A B, Lipinski M. Identification of a subset of normal B cells with a Burkitt's lymphoma (BL)-like phenotype. J Immunol. 1987;139:313–318. [PubMed] [Google Scholar]
- 21.Henderson S, Huen D, Rowe M, Dawson C, Johnson G, Rickinson A B. Epstein-Barr virus-coded BHRF1 protein a viral homologue of Bcl2 protects human B cells from programmed cell death. Proc Natl Acad Sci USA. 1993;90:8479–8483. doi: 10.1073/pnas.90.18.8479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hoffman G J, Lazarowitz S G, Hayward S D. Monoclonal antibody against a 250,000-dalton glycoprotein of Epstein-Barr virus identifies a membrane antigen. Proc Natl Acad Sci USA. 1980;77:2979–2983. doi: 10.1073/pnas.77.5.2979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Inman G J, Allday M J. Apoptosis induced by TGF-β1 in Burkitt's lymphoma cells is caspase-8-dependent but death receptor-independent. J Immunol. 2000;165:2500–2510. doi: 10.4049/jimmunol.165.5.2500. [DOI] [PubMed] [Google Scholar]
- 24.Inman G J, Allday M J. Resistance to TGF-β1 correlates with a reduction of TGF-β type II receptor expression in Burkitt's lymphoma and Epstein-Barr virus-transformed B lymphoblastoid cell lines. J Gen Virol. 2000;81:1567–1578. doi: 10.1099/0022-1317-81-6-1567. [DOI] [PubMed] [Google Scholar]
- 25.Jenkins P J, Binne U K, Farrell P J. Histone acetylation and reactivation of Epstein-Barr virus from latency. J Virol. 2000;74:710–720. doi: 10.1128/jvi.74.2.710-720.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kieff E. Epstein-Barr virus and its replication, P. 2343–2396. In: Fields B N, Knipe D M, Howley P M, editors. Fields virology. Philadelphia, Pa: Lippincott-Raven; 1996. [Google Scholar]
- 27.Kornberg A, Baker T. DNA replication, (2nd ed.) W. H. New York, N.Y: Freeman and Co.; 1992. [Google Scholar]
- 28.Liebermann P M, Hardwick J M, Sample J, Hayward G S, Hayward D. The Zta transactivator involved in induction of lytic cycle gene expression in Epstein-Barr virus-infected lymphocytes binds both AP-1 and ZRE sites in target promoter and enhancer regions. J Virol. 1990;64:1143–1155. doi: 10.1128/jvi.64.3.1143-1155.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.MacDonald I, Wang H, Grand R, Armitage R J, Fanslow W C, Gregory C D, Gordon J. Transforming growth factor-beta 1 cooperates with anti-immunoglobulin for the induction of apoptosis in group I (biopsy-like) Burkitt lymphoma cell lines. Blood. 1996;87:1147–1154. [PubMed] [Google Scholar]
- 30.Manet E, Gruffat H, Trescol-Biemont M C, Moreno L, Chambard P, Giot J F, Sergeant A. Epstein-Barr virus bi-cistronic mRNAs generated by facultative splicing code for two transcriptional transactivators. EMBO J. 1989;8:1819–1826. doi: 10.1002/j.1460-2075.1989.tb03576.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Marchini A, Tomkinson B, Cohen J I, Kieff E. BHRF1, the Epstein-Barr virus gene with homology to Bcl 2, is dispensable for B-lymphocyte transformation and virus replication. J Virol. 1991;65:5991–6000. doi: 10.1128/jvi.65.11.5991-6000.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Marshall W L, Yim C, Gustafson E, Graf T, Sage D R, Hanify K, Williams L, Fingeroth J, Finberg R W. Epstein-Barr virus encodes a novel homolog of the bcl2 oncogene that inhibits apoptosis and associates with Bax and Bak. J Virol. 1999;73:5181–5185. doi: 10.1128/jvi.73.6.5181-5185.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Massague J, Chen Y-G. Controlling TGF-β signalling. Genes Dev. 2000;14:627–644. [PubMed] [Google Scholar]
- 34.Miyashita E M, Yang B, Lam K M, Crawford D H, Thorley-Lawson D A. A novel form of Epstein-Barr virus latency in normal B cells in vivo. Cell. 1995;80:593–601. doi: 10.1016/0092-8674(95)90513-8. [DOI] [PubMed] [Google Scholar]
- 35.Parker G A, Touitou R, Allday M J. Epstein-Barr virus EBNA3C can disrupt multiple cell cycle checkpoints and induce nuclear division divorced from cytokinesis. Oncogene. 2000;19:700–709. doi: 10.1038/sj.onc.1203327. [DOI] [PubMed] [Google Scholar]
- 36.Pearson G R, Luka J, Petti L, Sample J, Birkenbach M, Braun D, Kieff E. Identification of an Epstein-Barr virus early gene encoding a second component of the restricted early antigen complex. Virology. 1987;160:151–161. doi: 10.1016/0042-6822(87)90055-9. [DOI] [PubMed] [Google Scholar]
- 37.Rickinson A B, Kieff E. Epstein-Barr virus. In: Fields B N, Knipe D M, Howley P M, editors. Fields virology. Philadelphia, Pa: Lippincott-Raven; 1996. pp. 2397–2446. [Google Scholar]
- 38.Rodrigues A, Armstrong M, Dwyer D, Flemington E. Genetic dissection of cell growth arrest functions mediated by Epstein-Barr virus lytic gene product, Zta. J Virol. 1999;73:9029–9038. doi: 10.1128/jvi.73.11.9029-9038.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rowe M, Lear A L, Croom-Carter D, Davies A H, Rickinson A B. Three pathways of Epstein-Barr virus gene activation from EBNA1-positive latency in B lymphocytes. J Virol. 1992;66:122–131. doi: 10.1128/jvi.66.1.122-131.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rowe M, Rowe D T, Gregory C D, Young L S, Farrell P J, Rupani H, Rickinson A B. Differences in B cell growth phenotype reflect novel patterns of Epstein-Barr virus latent gene expression in Burkitt's lymphoma cells. EMBO J. 1987;6:2743–2751. doi: 10.1002/j.1460-2075.1987.tb02568.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shimizu N, Takada K. Analysis of the BZLF1 promoter of Epstein-Barr virus: identification of an anti-immunoglobulin response sequence. J Virol. 1993;67:3240–3245. doi: 10.1128/jvi.67.6.3240-3245.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shimizu N, Tanabe-Tochikura A, Kuroiwa Y, Takada K. Isolation of Epstein-Barr virus (EBV)-negative cell clones from the EBV-positive Burkitt's lymphoma (BL) line Akata: malignant phenotypes of BL cells are dependent on EBV. J Virol. 1994;68:6069–6073. doi: 10.1128/jvi.68.9.6069-6073.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Speck S, Chatila T, Flemington E. Reactivation of Epstein-Barr virus: regulation and function of the BZLF1 gene. Trends Microbiol. 1997;5:399–405. doi: 10.1016/S0966-842X(97)01129-3. [DOI] [PubMed] [Google Scholar]
- 44.Swenson J J, Mauser A E, Kaufmann W K, Kenney S C. The Epstein-Barr virus protein BRLF1 activates S phase entry through E2F1 induction. J Virol. 1999;73:6540–6550. doi: 10.1128/jvi.73.8.6540-6550.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Takada K. Cross-linking of cell surface immunoglobulins induces Epstein-Barr virus in Burkitt lymphoma lines. Int J Cancer. 1984;33:27–32. doi: 10.1002/ijc.2910330106. [DOI] [PubMed] [Google Scholar]
- 46.Takada K, Ono Y. Synchronous and sequential activation of latently infected Epstein-Barr virus genomes. J Virol. 1989;63:445–449. doi: 10.1128/jvi.63.1.445-449.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tarodi B, Subramanian T, Chinnadurai G. Epstein-Barr virus BHRF1 protects against cell death induced by DNA-damaging agents and heterologous viral infection. Virology. 1994;201:404–407. doi: 10.1006/viro.1994.1309. [DOI] [PubMed] [Google Scholar]
- 48.Thorley-Lawson D A. EBV: schizophrenic but not disfunctional. Epstein-Barr Rep. 1999;6:158–163. [Google Scholar]
- 49.Tovey M, Lenoir G, Lours-Begon J. Activation of latent Epstein-Barr virus by antibody to human IgM. Nature (London) 1978;272:373–375. doi: 10.1038/276270a0. [DOI] [PubMed] [Google Scholar]
- 50.Urier G, Buisson M, Chamber P, Sergeant A. The Epstein-Barr virus early protein EB1 activates transcription from different responsive elements including AP-1 sites. EMBO J. 1989;8:1447–1453. doi: 10.1002/j.1460-2075.1989.tb03527.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.van Grunsen W M, van Heerde E C, de Haard H J, Spaan W J, Middeldorp J M. Gene mapping and expression of two immunodominant Epstein-Barr virus capsid proteins. J Virol. 1993;67:3908–3916. doi: 10.1128/jvi.67.7.3908-3916.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wade M, Allday M J. Epstein-Barr virus suppresses a G2/M checkpoint activated by genotoxins. Mol Cell Biol. 2000;20:1344–1360. doi: 10.1128/mcb.20.4.1344-1360.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Young L S, Lau R, Rowe M, Niedobitek G, Packham G, Shanahan F, Rowe D T, Greenspan D, Greenspan J S, Rickinson A B, Farrell P J. Differentiation-associated expression of the Epstein-Barr virus BZLF1 transactivator protein in oral hairy leukoplakia. J Virol. 1991;65:2868–2874. doi: 10.1128/jvi.65.6.2868-2874.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.zur Hausen H, O'Neill F J, Freese U K, Hecker E. Persisting oncogenic herpes-virus induced by tumor promoter TPA. Nature (London) 1978;272:373–375. doi: 10.1038/272373a0. [DOI] [PubMed] [Google Scholar]