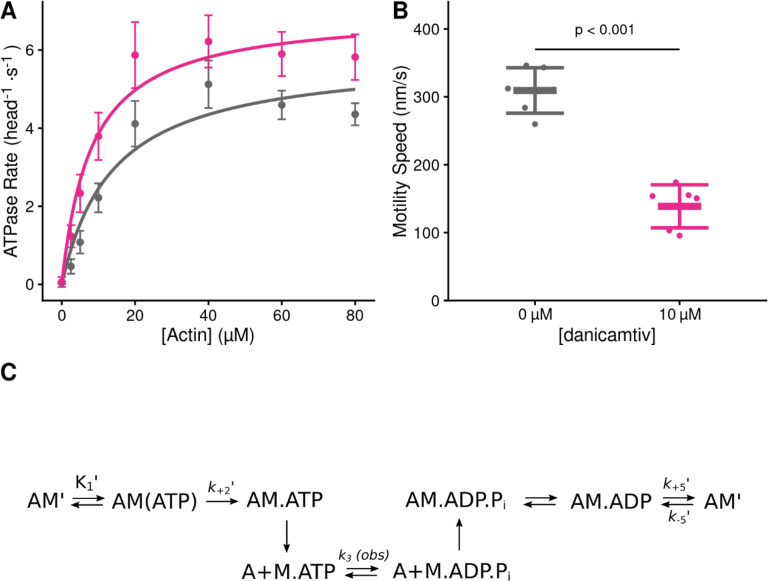
Figure 1. Steady state properties of β-cardiac myosin treated with danicamtiv.
A) The steady-state myosin ATPase rate was measured using the NADH coupled assay. The steady state myosin ATPase rate is plotted verses a function of actin concentration. Data were fitted by a hyperbolic function to calculate the maximal cycling rate (Vmax) and actin affinity (Km) with the Michaelis Menten equation. Treatment with 10 μM danicamtiv increased the maximal rate by ~1.2 fold from 5.9 to 7.0 s−1 (P = 0.028) and decreased the Km from 14.0 to 8.1 μM (P = 0.01). Each point represents the average rate from four independent trials with error bars showing the standard deviation. Statistical testing done using a 2-tailed T-test. Black = DMSO control. Pink = 10 μM danicamtiv. B) Speed of actin translocation in the unregulated in vitro motility assay. The addition of 10 μM danicamtiv decreased motility speed ~55% (P = 4×10−6). Thick horizontal lines show the average speed with standard deviation shown by the thin horizontal lines. Points represent the average speed of all filaments in a field of view for a single technical replicate measured across N = 3 independent experiments. Statistical testing done using a 2-tailed T-test. C) Scheme of myosin’s mechanochemical cross-bridge cycle. Myosin’s rate limiting step is actin attachment, so the predominant population of motors reside in the pre-working stroke M.ADP.Pi state during steady state cycling. The steady-state ATPase is thus limited by actin attachment (katt) which is rapidly followed by the mechanical working stroke and phosphate release. In vitro motility speed is limited by the ADP release rate (k+5’).