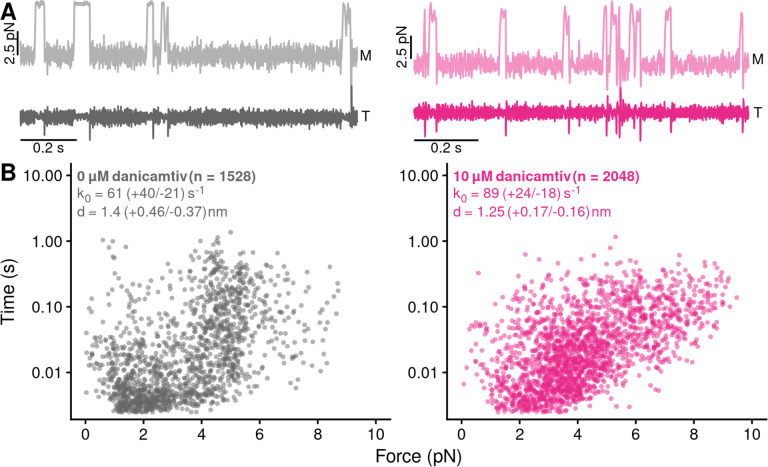
Figure 4. Danicamtiv does not alter myosin’s load-dependent detachment kinetics at 1 mM ATP.
Black = DMSO control. Pink = 10 μM danicamtiv. A) An isometric force clamp was used to maintain actin at an isometric position during myosin binding interactions. To do this, the motor bead (M) was moved to hold the transducer bead (T) at an isometric position. Data traces are shown. B) Plots of actomyosin attachment duration versus the average resistive force applied during the binding event. Data are exponentially distributed at each force. Each point represents an independent actomyosin binding interaction. The data were fitted with the Bell equation using maximum likelihood estimation and 95% confidence intervals were calculated for each parameter by bootstrapping. The detachment rate in the absence of load, k0, was not different between control and 10 μM danicamtiv, 61 (−21/+40) vs 89 (−18/+24) s−1 (P = 0.17). These values are consistent with our measurements of the rates of ADP release from stopped-flow experiments. The distance to the transition state, d, which measures the load-sensitivity of the detachment rate, was not different between control and 10 μM danicamtiv, 1.40(−0.37/+0.46) vs 1.25 (−0.16/+0.17) nm (P = 0.43).